Direct-to-Consumer (DTC) drug advertising has become a favorite topic of discussion in recent years. These advertisements, which are aimed at consumers rather than healthcare professionals, have been credited with increasing patient awareness and demand for certain drugs. However, they have also been criticized for potentially leading to overdiagnosis and overtreatment.
Participating in the current sprint of trends within pharmaceutical companies, total spending on marketing is known to be more than their research budget. Globally, $1.42 trillion was spent worldwide on marketing. In the USA, $486.62 billion was spent in 2021 to market drugs to physicians; in Canada, $29,305.1 million was spent in 2022. When these numbers were broken down, 56% were free samples, 25% were pharmaceutical sales representative "detailing" (promoting drugs directly to) physicians, 12.5% were direct-to-user advertising, 4% were on detailing to hospitals, and 2% were on journal ads2. Sometimes negative marketing practices can affect both patients and the healthcare profession.
Types of Drug Promotional Outreach
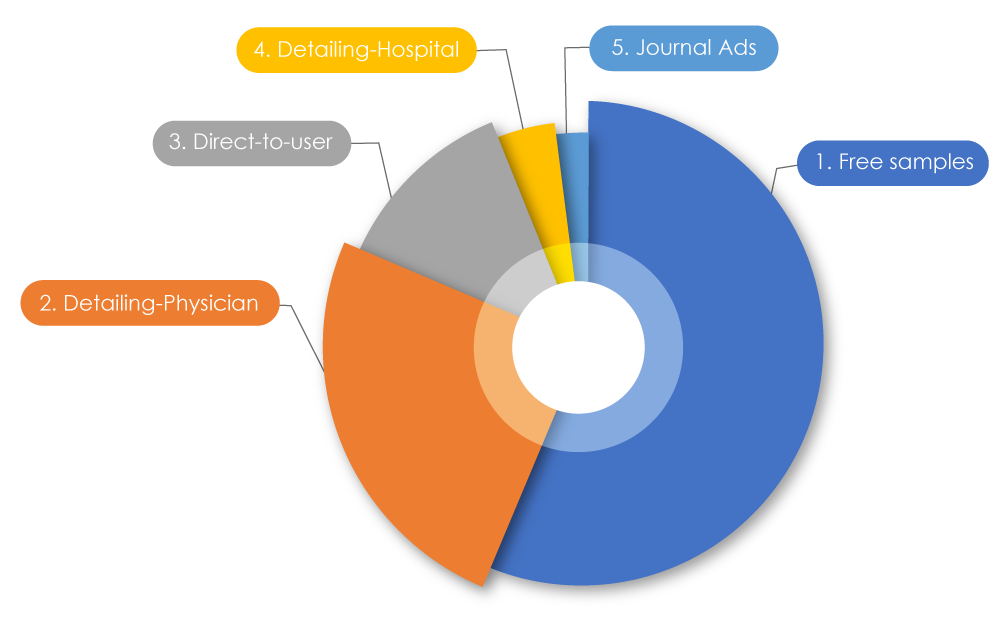
Source: Pharmaceutical marketing - Wikiwand
Pharmaceutical Advertisement to the General Public
Restrictions are imposed on advertising prescription drugs to the general public, also commonly referred to as direct-to-consumer (DTC) advertising. For promoting prescription drugs to the public, the communication should meet certain fundamental requirements:
- On-label consistency of advertising and promotion of prescription drugs relies on the intended use for which the product is approved by the FDA (the drug’s FDA-approved labeling). The labeling provides information about how to use the product safely and effectively for the approved indication. Advertising and promotion that are inconsistent with the FDA-approved labeling are regarded as unlawful “off-label” promotions.
- Prescription drug promotion and advertising require a fair balance between product benefits and risks, ensuring that such information appears comparable in depth, detail, and context
- Claims should be supported by adequately substantial evidence or clinical experience
- Truthful and not misleading - If prescription drug advertising and promotion are false or misleading in any particular, it will be considered misbranded under the Federal Food, Drug, and Cosmetic Act (FDCA) and subject to enforcement
- Usage of consumer-friendly language, avoiding the use of technical language, scientific terms, and medical jargon in consumer-directed advertising and promotion. It must adhere to the product’s approved labeling or monograph
Information Contained in Pharmaceutical Advertising for the General Public
Consumer-directed prescription drug advertising and promotion must contain the following core elements, as required by the FDCA and Food and Drug Administration (FDA) regulations.
- Proprietary and established names should be as specified in FDA regulations
- It should include the quantitative amount of ingredients of the advertised drug
- It should include a “brief summary” that discloses each side effect, warning, precaution, and contraindication. Mainly focused on the important risk information rather than an exhaustive list of product-related risks. Most of the risks are presented in a way that is most likely to be understood by consumers
- Product major risks should be presented in a clear, conspicuous, and neutral manner as a “Major statement”
- Reporting disclosure statements for adverse events for DTC advertisements must include the following MedWatch statement printed in conspicuous text: “You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call +1–800-FDA-1088”
- Reminder labeling and advertising are exempted from the general requirements. Importantly, reminder labeling and advertising are not permitted for a prescription drug with a boxed warning in its FDA-approved labeling
For DTC Drug Advertising, Contact Us
For DTC Drug Advertising
Restrictions on Interactions Between Patients or Patient Organizations and Industry
Interactions between pharmaceutical companies and patients/patient organizations are permitted only in a few countries, such as the United States but with limitations, such as:
- Must be on-label/CFL
- Fair balanced
- Adequately substantiated and
- Not otherwise false or misleading
In addition, interactions with customers must not implicate the Anti-Kickback Statute by inducing patient organizations or patients to recommend or use the advertised product.
In the UK, advertising of DTC to the public is permitted for pharmaceuticals but excludes children. The advertising of prescription-only medicines to the public is prohibited. Advertisements should include the name of the product and the name of the active ingredient. Samples of products or vouchers that permit the consumers to obtain the product for free or at an unreasonably low price are not permitted.
In Germany, advertising to the public is permitted for pharmaceuticals exclusively for children under the age of 14. The advertising of prescription-only medicines to the public is prohibited. But there are also special rules for the advertising of medicinal products outside professional circles. These special rules are mainly stated in the Advertising of Medicines Act (Heilmittelwerbegesetz, "HWG") and were amended by the German Act on Pharmaceuticals (Arzneimittelgesetz – "AMG") to be in line with Directive 2001/83/EC4.
Debating the Possibility of Drug Advertising in the Digital Era
Proponents of DTC drug advertising argue that it empowers patients by providing them with information about treatment options. They also point out that it can lead to increased demand for drugs, which can be beneficial for pharmaceutical companies.
On the other hand, critics of DTC drug advertising argue that it can lead to overdiagnosis and overtreatment. They claim that these advertisements often exaggerate the benefits of drugs while downplaying their risks, leading patients to ask their doctors for medications they may not need. Additionally, they state that it can lead to increased healthcare costs, as patients may be prescribed more expensive brand-name drugs rather than cheaper generic alternatives.
Despite the ongoing debate, DTC drug advertising is here to stay. The FDA has issued guidelines for this sub-type of advertising, which include requirements for risk disclosure and a fair balance between benefits and risks. In conclusion, DTC drug advertising has the potential to educate patients and increase demand for certain drugs. However, it is also important to consider the potential downsides and to be aware of the limitations of the information provided in these advertisements. As always, it's important to consult with your healthcare provider before taking any medication. Reach out to Freyr experts for end-to-end compliance!