During the period when Covid struck hard to the whole world, we were searching for only words which could give us hope to survive such as “Efficacy” and “Risk”. Vaccines were looked at for their efficacy and to understand about their risks by everyone. Pre-Covid, have we ever searched for the “Efficacy or Risks association with” Paracetamol or Aspirin……….! We guess most of us will say- NO!
Every choice to take a medicine involves thinking through its helpful effects as well as the possible unwanted effects. Different drugs pose different threats to life such as dependence and addiction, injury and accidents, health problems, sleep issues, and more. So, consumers should be very aware of the “Risks” associated with any drug to help themselves!
What does Quantitative efficacy sound like?
It demonstrates the effectiveness of a product in treating a specific medical condition. And should be presented clearly and explicitly to help consumers make informed decisions. Quantitative efficacy information in Direct-to-consumer (DTC) materials includes clinical trial results, comparative effectiveness, and numeric data related to patients.
For Market Complaint DTC Promotional Communication. Contact Us
For Market Complaint DTC Promotional Communication
What is Risk information, and should it be presented in DTC materials?
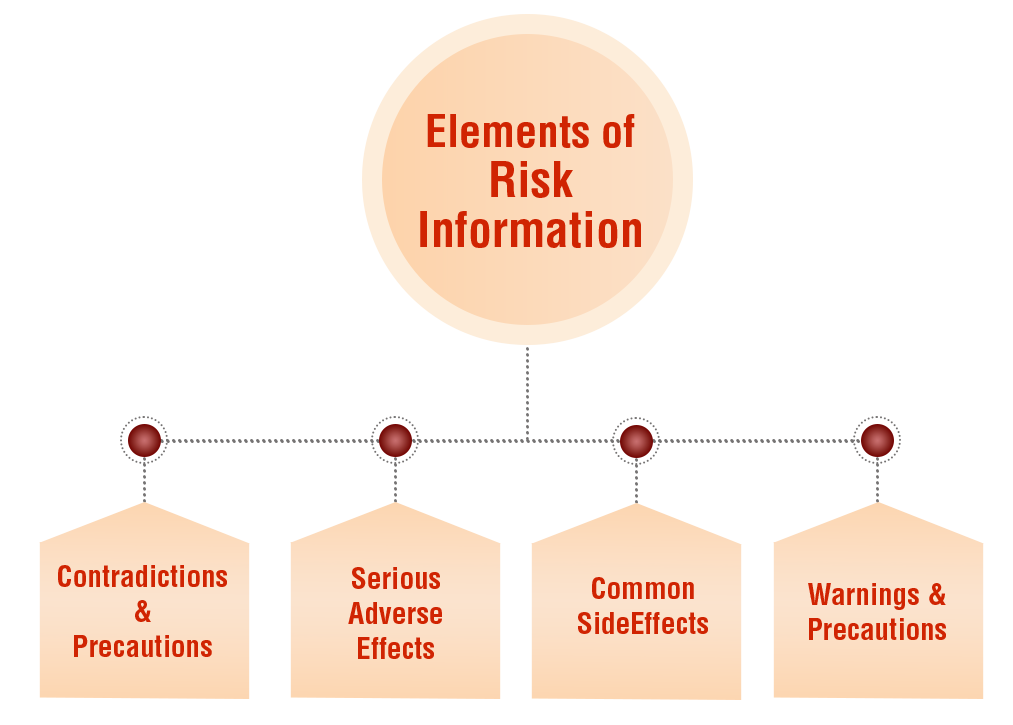
In addition to highlighting the benefits of a product, DTC materials must also provide comprehensive risk information. This includes informing consumers about potential side effects, contraindications, precautions, and interactions with other medications. A few states below are the parts of the risk information.
Recent Guidance on Direct-to-Consumer Advertisements [Efficacy + Risk]
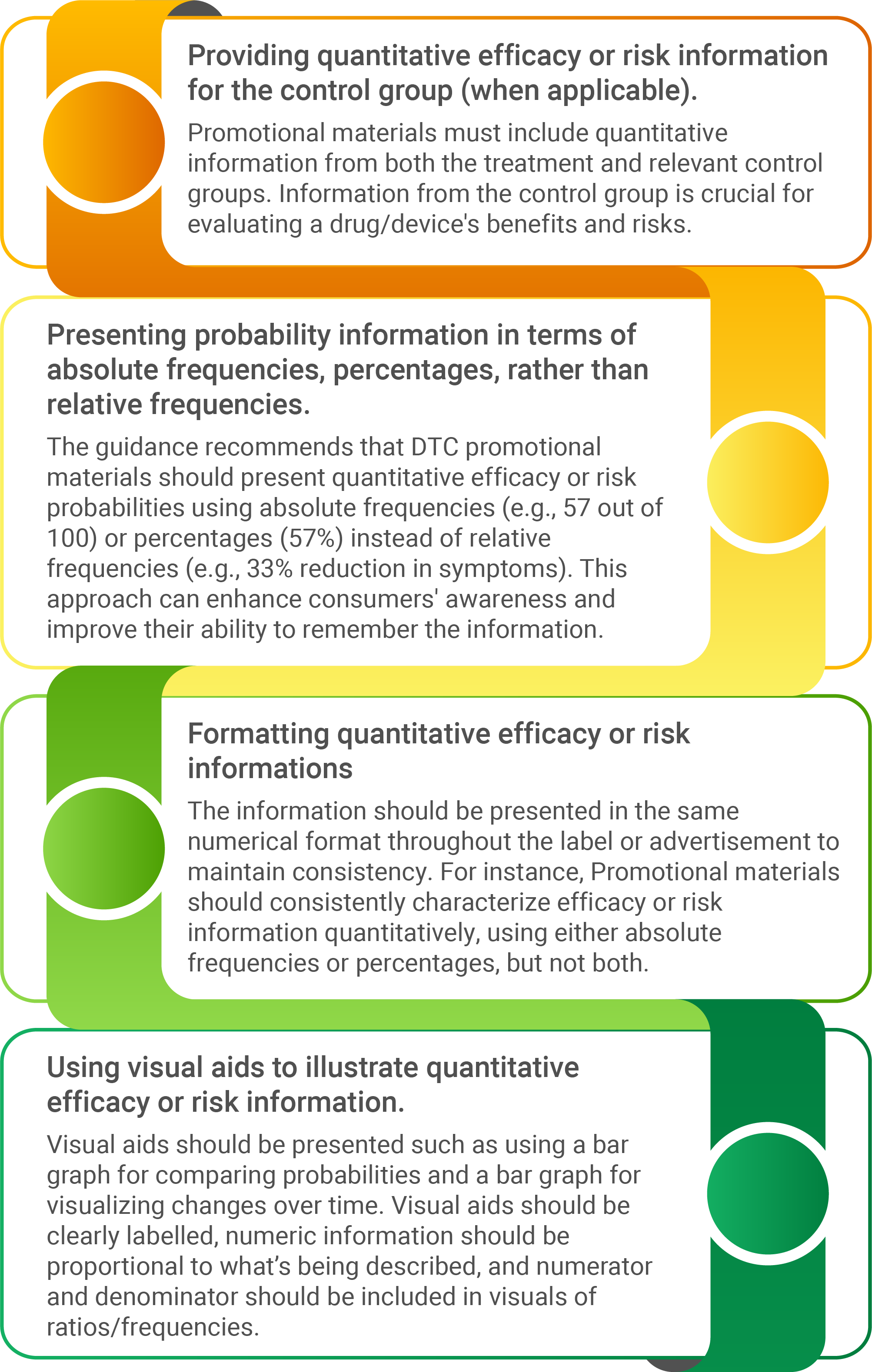
Keeping this new development in consumers’ thoughtfulness and digitalization; the FDA has released a recent guideline on “Presenting Quantitative Efficacy and Risk Information in Direct-to-Consumer (DTC) Promotional Labelling and Advertisements." The guidance offers a valuable recommendation on DTC advertisements presenting efficacy and risk information in quantitative terms.
The guidance unfolds the following connotation for presenting quantitative efficacy and risk information in DTC promotional communications:
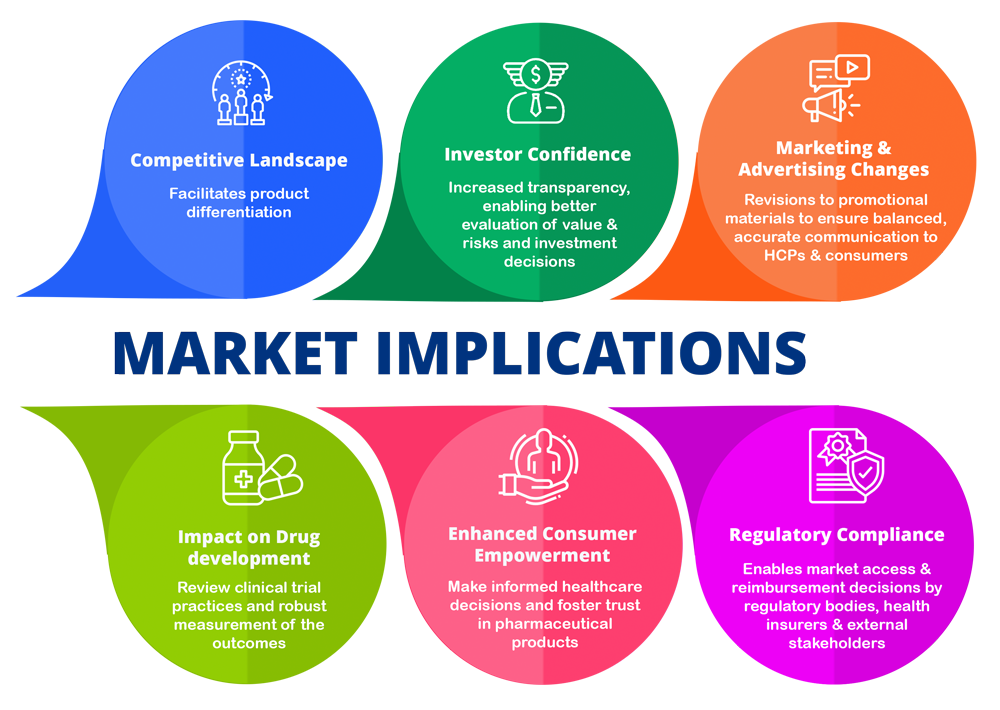
Market implications
This final guidance can bring various implications for the market and consumer patients-
Not following the FDA guidance may result in -
- Companies may be fined or receive warning letters from the FDA. In most industries, inaccurate or misleading marketing may get banned. But in the world of pharmaceuticals, biotech, and life sciences, the hefty fine can easily exceed $100 million.
- It could ruin the company's reputation if any of its brands are in newspapers across the country for the wrong reasons. And suddenly the partners and patients would want to take their business elsewhere.
Freyr, with more than a decade in the regulatory industry and a global presence with a pool of experts, has a correct understanding of how this guidance will impact the industry and the immediate next steps. Partner with us to stay compliant always.