
Introduction
The European Directorate for the Quality of Medicines and Healthcare recently announced an update on their (CEP) process, called promises to be more efficient, transparent, and user-friendly than its predecessor. In this blog, we will explore the benefits of and the role of Regulatory vendors in helping pharmaceutical companies navigate this new system.
CEP 2.0: CEP of the Future
The current ensure the quality and safety of medicinal products. However, the lack of harmonization in fulfilling the CEP requirements across different regions and inefficiency in the have initiated the upgrade. For example, there have been cases where companies have submitted to and waited for several months without any feedback. As a result, the process has become time-consuming and opaque for the stakeholders.
CEP 2.0 has addressed these challenges across the system. According to the EDQM, this version of the CEP ensures a more streamlined and efficient process with increased transparency amongst the stakeholders.
Changes in
CEP 2.0 presents several changes to the process. A few of them are as follows:
A. Fully digital process: This change indicates that pharma companies will no longer be required to provide hard copies of their CEP application documents. Instead, they can submit their applications electronically through the EDQM’s CEP submission portal.
B. Introduction of an online platform: It allows companies to track the progress of their applications in real time. It also provides access to all communications between the EDQM and the applicant, including any questions or concerns raised during the review process.
C. Pre-submission phase: This helps applicants identify any issues or concerns with their CEP application before submitting it for review. An applicant can submit a pre-submission inquiry, which will be reviewed by the EDQM within twenty (20) working days. The EDQM will provide feedback on the CEP application's suitability and identify any potential issues to be addressed by the applicant.
The changes related to implementation are highlighted in the infographic below:
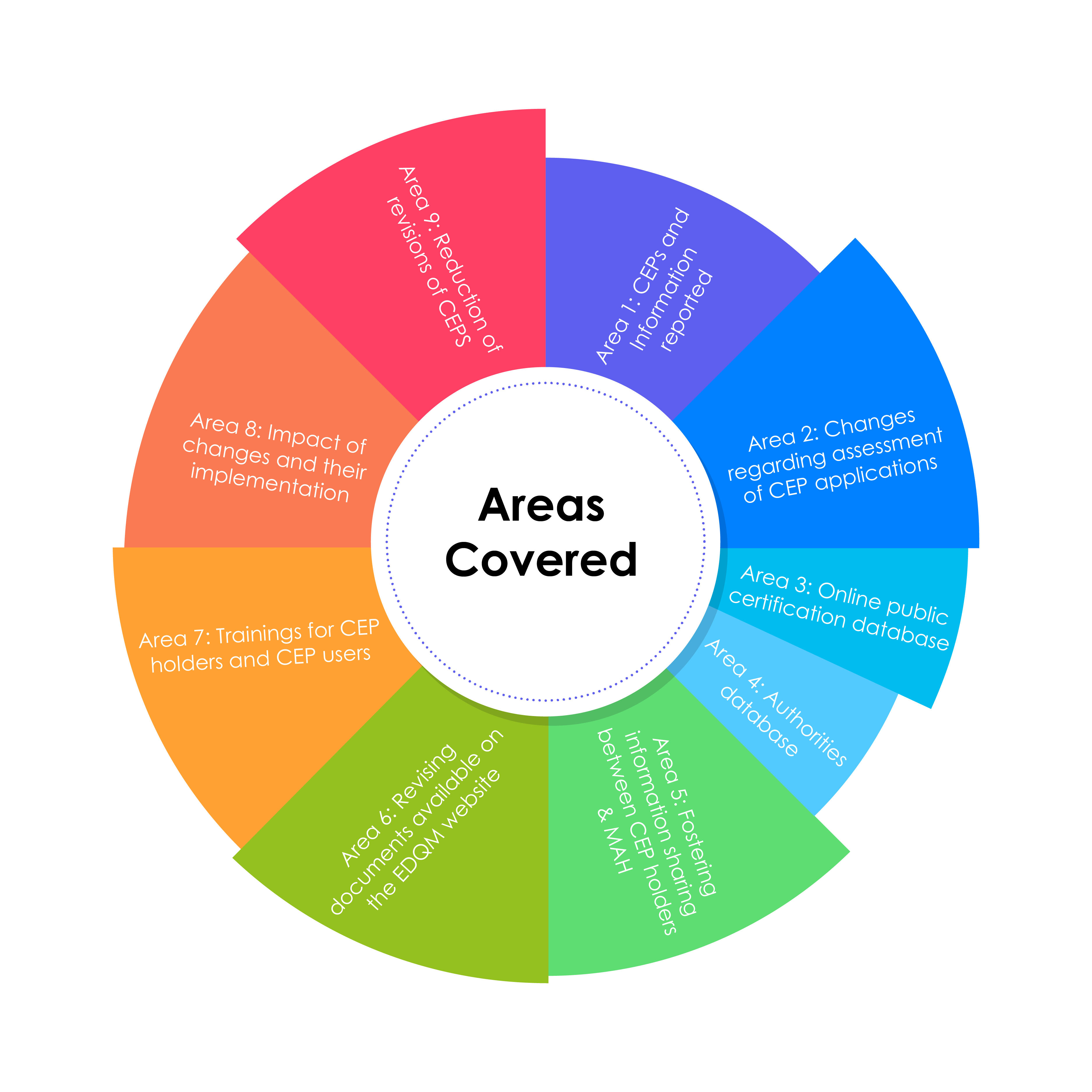
Areas Covered by EDQM’s CEP 2.0: The CEP of the Future
Challenges in Certification Process
The new process poses significant challenges to pharmaceutical manufacturers seeking certification. Navigating the complexities of the process requires specialized knowledge and expertise that many companies may lack. A few of the challenges are:
- Expertise pre-requisite: CEP 2.0 introduces a fully digital application and review process of the applicant’s CEP, which will require a system expert.
- Efficiency obligation: The new system will feature a streamlined assessment process, which will reduce the EDQM’s time to assess CEP applications and issue certificates. This challenges the pharmaceutical companies to have an efficient system to fulfill the requirements.
- Demand for compliance: CEP 2.0 processes ensure that pharmaceutical companies comply with all the related regulations. On the other hand, pharma companies need to understand compliance requirements on new data protection measures, information submission norms, and newly introduced regulations.
In such endeavors, Regulatory vendors can play a vital role in helping manufacturers navigate the new process. Partnering with experienced Regulatory vendors can help ensure a successful outcome through the timely approval of CEPs.
CEP 2.0 promises to be a game-changer for pharmaceutical companies looking for EU market entry. The new system offers several benefits over its predecessor, including a simplified application of the CEP process, a streamlined assessment process, improved transparency, and enhanced data protection measures. Regulatory vendors can play a pivotal role in helping pharmaceutical companies navigate this new system by providing expertise, efficiency, and compliance guidance.
At Freyr, our team can ensure the compilation, review, and submission of CEP to be hassle-free. The right Regulatory partner for CEP submission can sail your organization through any roadblocks toward a fool-proof and compliant journey. Consult Freyr to learn more about our expertise.









