
A 510(k) or a pre-market notification is a submission made to the United States Food and Drug Administration (US FDA) to demonstrate that the device to be marketed is safe and effective, that is, substantially equivalent to a legally marketed or a predicate device. The following are the three (03) types of 510(k) that a medical device manufacturer can submit:
- Traditional
- Abbreviated
- Special 510(k)
In this blog, we will be examining the cases under which your application would qualify for the second type, an abbreviated 510(k), as per the US FDA requirements.
An abbreviated 510(k) is used for showing substantial equivalence to a recognized standard, special control, or guidance using a Declaration of Conformity (DoC). In an abbreviated submission, manufacturers show substantial equivalence to recognized standards based on the usage of guidance documents or DoCs, instead of a predicate device, to facilitate the US FDA’s review. Below is the flowchart for determining the substantial equivalence for an Abbreviated 510(k) application.
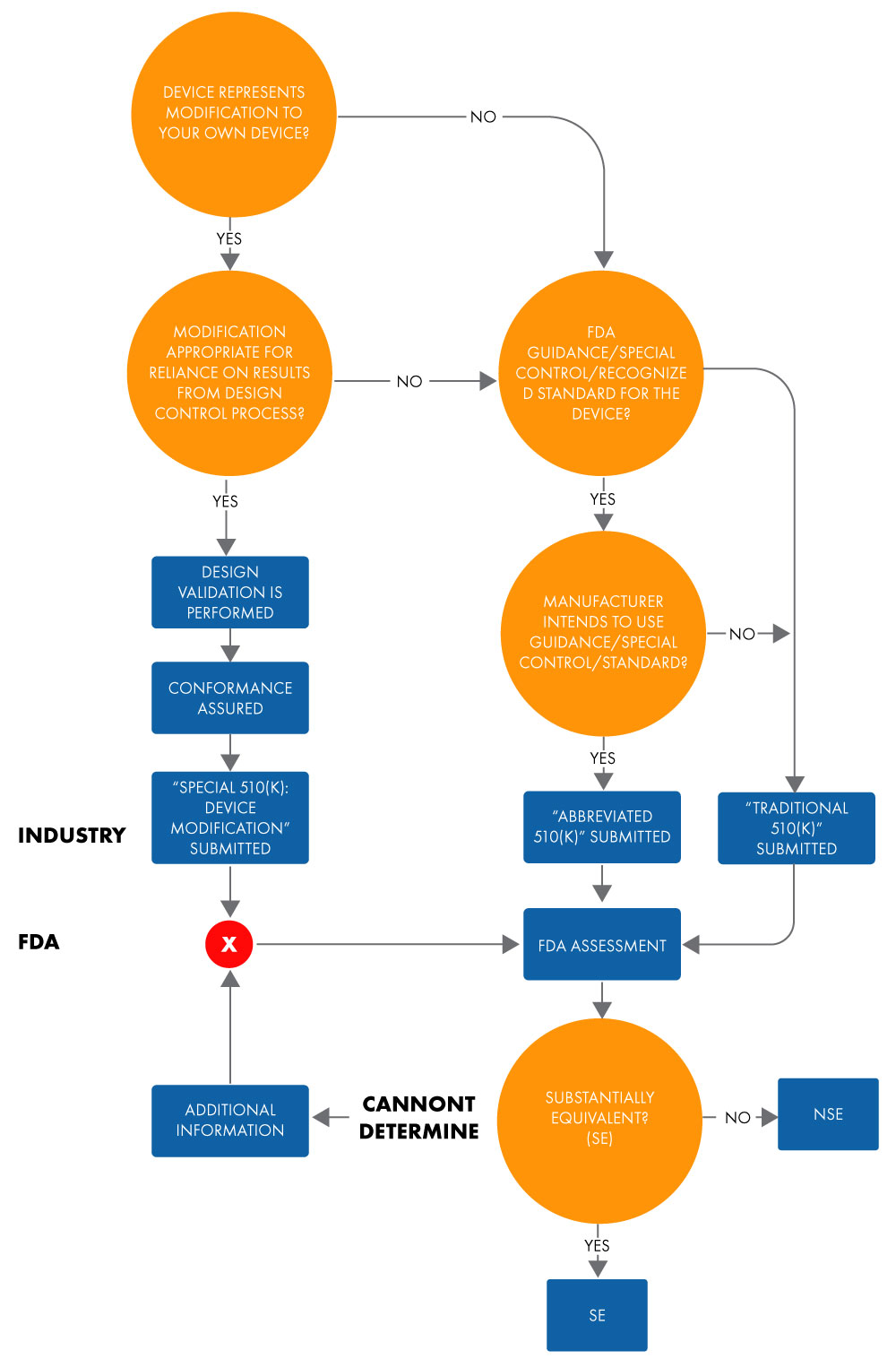
Figure 1: Intent to Market Device Via 510(k)
The term “abbreviated” suggests that this type of 510(k) approval process is shorter. However, this is not entirely true. It takes as much time as a traditional 510(k) approval. The same holds true for documentation and costs. Moreover, the format for both traditional and abbreviated 510(k), in terms of chapterization and structure, are similar.
While submitting an abbreviated 510(k) you should rely on the identified elements in 21 CFR 807.87 (traditional 510[k] submissions). You may choose to submit an abbreviated 510(k) when the submission relies on the following:
- FDA Guidance Document(s): While submitting an abbreviated 510(k), you should include a summary report that outlines the adherence to the relevant guidance document and how it was used during the development and testing of the device.
- Demonstration of Compliance with Special Controls for the Device Type: You should comply with special controls such as performance standards, Post-marketing Surveillance (PMS), patient registries, development and dissemination of guidelines, recommendations, etc., which provide reasonable assurance of the safety and effectiveness of the device. An abbreviated 510(k) submission that relies on a special control(s) should include the following. A summary report that describes adherence to the special controls and how they were used during the development and testing of the device.
- How the special controls was used to address a specific risk or an issue.
- Information describing any deviations from the specific controls and the manufacturer’s attempts to comply with them.
- Voluntary Consensus Standard(s): You are required to provide a DoC to the recognized standard for an abbreviated 510(k) submission that relies on it. A DoC should include the following:
- The name and address of the applicant/sponsor who is responsible for the DoC.
- Details of product/device identification, including product codes, device marketing name, model number, and any other unique product identification data specific to the DoC in question.
- A statement of conformity.
- A list of standards for which the DoC is applicable, including the option(s) selected for each standard, if any.
- The US FDA recognition number for each standard.
- The date and place of issuance of the DoC.
- The signature, printed name, and function of the sponsor responsible for the DoC.
- Any limitation(s) on the validity of the DoC (for instance, for how long the declaration is valid, what was tested, concessions made about the test outcomes, etc.)
In conclusion, an abbreviated 510(k) is a useful way for device manufacturers to demonstrate substantial equivalence to recognized standards or special controls using a DoC. To qualify for an abbreviated 510(k), device manufacturers must provide a summary report explaining their adherence to the relevant guidance documents, demonstrate compliance with special controls, and provide DoCs to recognized standards. It is, however, important to note that the approval process, documentation, and cost of an abbreviated 510(k) are similar to those of a traditional 510(k).
Does your medical device qualify for an abbreviated 510(k) application? For any assistance in filing your abbreviated 510(k) application, reach out to our Regulatory expert. Stay informed! Stay compliant!









