
The Malaysia pharmaceutical market is a growing hub in Southeast Asia, offering major opportunities for global companies. With a rising demand for quality healthcare and an increasingly structured regulatory framework, the pharmaceutical market in Malaysia is becoming an attractive destination for both generic and innovative drug manufacturers.
Malaysia Market Overview: A Promising Landscape in the Malaysia Pharmaceutical Market
Both generics and New Chemical Entities (NCEs) have strong prospects in the Malaysia pharmaceutical market, a rapidly developing industry in Southeast Asia. The nation presents a dynamic environment for pharmaceutical companies due to its growing population and increasing healthcare demands. The healthcare industry is a lucrative market for pharmaceuticals since it is well-established and has first-rate medical facilities.
The pharmaceutical market Malaysia is characterized by rising demand for novel medicines, especially in therapeutic areas like diabetes, cardiovascular disease, and oncology. The Regulatory environment has grown more organized, with precise rules for product approval and market entry, while the government keeps concentrating on enhancing public healthcare and medication access.
Registration Process in Malaysia:
The process for registering pharmaceutical products in Malaysia is governed by the National Pharmaceutical Regulatory Agency (NPRA), which is responsible for ensuring the safety, efficacy, and quality of pharmaceutical products.
The NPRA requirements for registration of pharmaceutical products are aligned with the guidelines and recommendations for quality, safety and efficacy of the World Health Organization (WHO) or other internationally accepted standards such as International Conference of Harmonization (ICH).The key steps in the registration process are as follows:
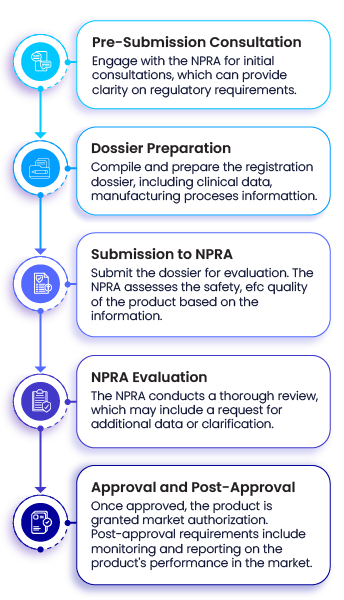
Basic Regulatory Requirements:
A pharmaceutical product must fulfill several important Regulatory conditions to be registered in Malaysia:
- Good Manufacturing Practice (GMP): To guarantee that the product satisfies quality requirements, the manufacturing facilities need to be accredited by the PIC/S Participating Authorities.
- Clinical Data: To prove safety and effectiveness, NCEs need clinical trial data. Bioequivalence must be demonstrated for generic products.
- Labeling and Packaging: Local laws must be followed when labeling products, especially those pertaining to language and safety information.
- Product Monograph: A comprehensive description of the product's ingredients, dosage, and usage guidelines is necessary.
- Local Representation: Usually, a local agent or representative must serve as the NPRA's point of contact. The applicant for product registration, known as the Product Registration Holder (PRH), must be a locally incorporated company, corporate or legal entity, with permanent address and registered with the Companies Commission of Malaysia (SSM) (with business scope related to health/ pharmaceutical product).
Freyr’s Case Study: Supporting a Taiwan-Based Pharmaceutical Company in Market Entry:
Client Overview: A leading Taiwan-based pharmaceutical company sought end-to-end regulatory support from Freyr to launch both generic and NCE medicinal products in the Malaysia pharma market. Their goal was to ensure timely market entry while overcoming regulatory complexities.
Project Details: Freyr’s engagement includes a full spectrum of regulatory services:
- Regulatory Strategy and Approach: A strategy that complies with NPRA regulations and is customized to the demands of the customer was developed for the product launch in Malaysia.
- Technical Review and Gap Analysis: A comprehensive examination of the registration documents was carried out in order to find any gaps and make sure they complied with NPRA's requirements.
- Compilation and Submission: Made sure that all required documentation was complete and in compliance with regulatory standards before compiling and submitting the registration dossier to NPRA.
- Follow-up with NPRA: Oversaw continuing correspondence with NPRA to resolve any questions or further requests, accelerating the approval procedure.
Outcome: With Freyr's assistance, the client was able to successfully complete the Malaysian registration process. The items were approved quickly, enabling a seamless market entry, filling in any technical gaps, and making sure the right paperwork was submitted. This case highlighted Freyr’s strength in regulatory affairs and its role in navigating the pharmaceutical market in Malaysia effectively.
The customer was able to concentrate on their primary business operations thanks to Freyr's end-to-end support, which made sure that the entire Regulatory process—from dossier preparation to final approval—went smoothly.
Ready to Enter Malaysia’s Thriving Pharmaceutical Market?
The Malaysia pharmaceutical market offers immense growth potential. However, navigating the Regulatory framework requires a well-structured strategy, precise documentation, and local insight.
By partnering with experienced regulatory service providers like Freyr, pharmaceutical companies can streamline their entry into the Malaysia pharma market, address technical gaps, and gain faster approvals—unlocking new opportunities in this vibrant region.









