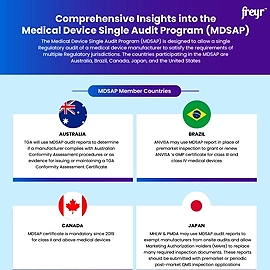
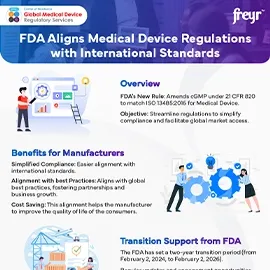
Supplier evaluation and qualification are critical processes in the medical device industry due to the high stakes involved in product safety, Regulatory compliance, and patient health. Here's why these processes are essential
- Regulatory compliance with standards like FDA 21 CFR Part 820, which mandates purchasing controls to ensure suppliers meet specified requirements, and ISO 13485, which requires documented procedures for supplier selection, evaluation, and re-evaluation.
- Risk Management: Identifies and mitigates supplier-related risks early in the supply chain.
- Traceability: Enables quick root cause analysis and corrective actions during issues or recalls.
- Audit Readiness: Maintains complete documentation and performance records for inspections.
Our Global Market Expansion Services for SaMD
Freyr plays a strategic role in helping medical device companies establish and maintain a robust supplier evaluation and qualification process.
Our services include-
Regulatory Guidance
Our Quality and Regulatory experts bring in-depth knowledge of global Regulatory standards such as FDA 21 CFR Part 820, ISO 13485, and EU MDR, ensuring that supplier qualification processes align with current compliance requirements.
Independent Supplier Audits
We conduct thorough supplier audits- either remotely (Desktop audits) or on-site, to assess quality systems, manufacturing capabilities, and compliance readiness. These audits provide objective insights into supplier performance and reliability.
Risk-Based Evaluation Frameworks
Help implement risk-based evaluation models and frameworks that prioritize suppliers based on the criticality of their products or services.
Documentation and Compliance Support
Assist in developing and maintaining essential documentation, including:
- Supplier qualification records
- Audit reports
- Approved supplier lists
- CAPA documentation related to supplier issues
Ongoing Supplier Monitoring
Support continuous supplier oversight through:
- Periodic re-evaluations
- Performance scorecards
- Feedback mechanisms for continuous improvement