Freyr’s Quality Management System (QMS) consultancy services help medical device manufacturers establish and maintain systems that meet global Regulatory standards. These services ensure product quality, patient safety, and compliance with regulations like-
- ISO 13485:2016
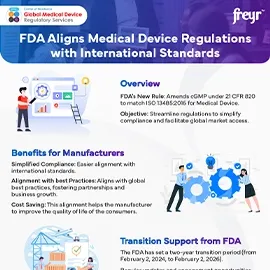
- US FDA 21 CFR 820
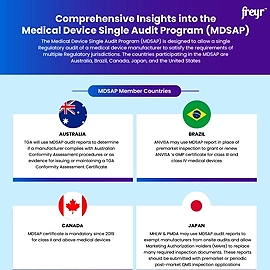
- Medical Device Single Audit Program (MDSAP)
- Korea Good Manufacturing Practice (KGMP)
- Brazilian Good Manufacturing Practice (BGMP)
- Indian MDR 2017
- Quality System Documentation (QSD), Taiwan
- Ministry of Health, Labor and Welfare (MHLW), Japan MO169
Our Core Services Include:
- QMS Design & Implementation
- Regulatory Compliance Support
- Audit & Gap Assessments
- Risk Management
- Training & Education