Medical Copywriting Services - Overview
Freyr brings a unique synergy of complementary expertise in Medical and scientific writing (content curation), creative scientific studio (graphic design and animations), Content Marketing editors (tailored content adaptation for Generative Engine Optimization and traditional SEO), and ‘Ad Promo’ Regulatory Compliance (MLR Reviews) for all your advertisement and promotional needs throughout the product lifecycle.
Whether it be medicinal products, medical devices, Nutraceuticals, or Dietary Supplements, it is crucial that product promotions effectively strike the right balance between different complex nuances such as,
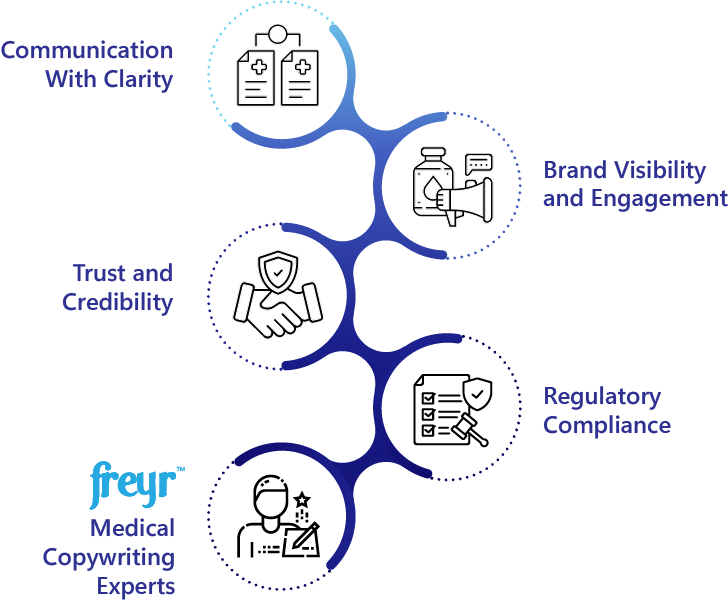
- Effective communication with Clarity - Translating complex medical concepts, scientific data, and clinical trial outcomes into easily comprehensible formats for HCPs and Patients.
- Enhance brand visibility and engagement by reassuring visual creatives and engaging compelling narratives on brand awareness and distinctive value propositions to generate interest in disease state, health issues, treatment options, and the company’s products.
- Establishing Trust and Credibility - Ensuring evidence-based substantiation, no misleading Claims, scientifically accurate, well-balanced presentation of risks alongside benefits to avoid misinformation.
- Ensuring Regulatory Compliance - Promotional communications adhere to Health Authority (HA) requirements and Industry Ethical compliance codes.
Achieving this right balance in promotional communication requires niche, multifaceted expertise to curate a perfect harmony of the Art of Creative Content Marketing with Clinical Precision and Scientific Accuracy.
At Freyr, our team of multifaceted Medical Copywriting experts can transform your Advertisements, Brand Promotions, scientific research, and Targeted Campaigns into regulatory-compliant, rewarding collaterals for target audience engagement over digital media channels and print formats.
Medical Copywriting Services
As a trusted medical copywriter team, we specialize in crafting scientific stories and promotional content that connect with HCPs, patients, and caregivers across digital and print formats. Our portfolio features numerous medical copywriting examples that showcase our range across therapeutic areas and span a wide array of advertisement and promotional assets for Print, Digital, Television, and Radio media.
Content Curation
- Curating promotional materials for various marketing channels, including websites, brochures, social media, and email campaigns.
- Content transcreation and adaptation for local markets.
- SEO-optimized content tailored to resonate with HCPs and Patients.
Creative Design
- Scientific Design of Bioimages, Scientific illustrations, Infographics, Brochures, Flyers, REMS materials, IFUs, eLearning modules, Standees, Banners & Posters for Events/Conferences/Symposia.
- MoA/IFU/Awareness Videos, 2D Animations.
MLR Review and HA Consultation
- Claims Substantiation, Fact Checks, and Reference Validation
- Medical and Regulatory (MLR) compliance reviews
- Regulatory compliance with country-specific guidelines established by Regulatory authorities for 120+ countries, such as the FDA, EMA, MHRA, TGA, HSA, PMDA, ANVISA, Health Canada, Medicines Australia, Medicines New Zealand, and others.
- Compliance with industry body ethical codes worldwide for 120+ countries, such as PAAB, ABPI, PAGB, ASA, PhRMA, IFPMA, and PSA, but not limited to.
- Advertisement and Promotional labeling Submission to the Health Authority, such as OPDP/APLB Form 2253 to the US FDA in manual and eCTD formats.


Content Marketing Execution
- Specialized content distribution services to strategically position promotional content across various digital channels such as Websites, Emailers, and social media.
- Curate HCPs and patient-targeted campaigns for social media marketing and email campaigns, and promote organic website traffic with A/B testing.
- Maximize visibility and engagement through effective GEO (Generative Engine Optimization), Traditional SEO, and feedback loops.
- Organize Webinar kits such as emailers, invites, moderator scripts, speaker bios, and post-webinar summaries.
- Content playbooks for sales reps and the field force.
- Performance-Driven Content Execution, such as Content tagging and analytics for better tracking, Content refresh cycles based on usage metrics and sales feedback, Content audit, and Gap analysis (based on therapeutic areas, product lifecycle, HCP preferences).
- Support for Annual/quarterly content calendars with campaign themes, channels, and KPIs, considering Persona-based messaging for different HCP segments and Omnichannel content mapping aligned with the sales funnel.
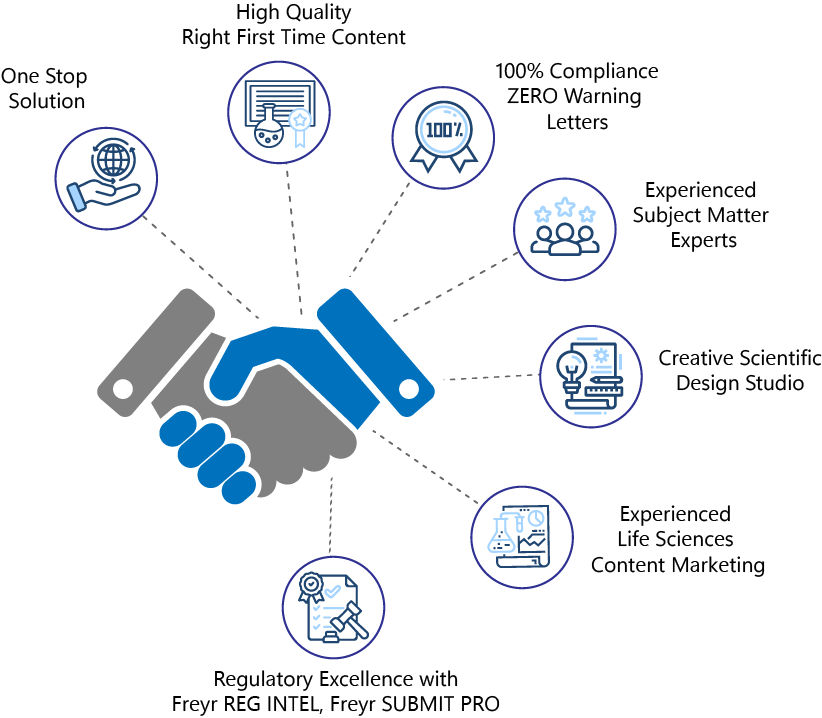
Why choose Freyr as your Life Sciences Content partner?
- One-Stop Shop for all your advertising and Promotional needs. We provide everything from Content Curation, Creative Design, MLR Reviews, Regulatory Compliance, and HA Submission to Digital Content Marketing services under One (1) umbrella.
- High-quality ‘Right First Time’ content tailored to resonate with various target audiences.
- Zero warning letters, 100% MLR compliance-backed by our seasoned team of medical copywriters and Regulatory reviewers.
- Expert blend of subject matter experts in copywriting for healthcare, scientific publications, and digital marketing.
- Cutting-edge Creative Scientific Design studio for Life Sciences Marketing and Medical Communication.
- Regulatory Excellence powered by Freyr Reg Intel and Freyr SUBMIT Pro for up-to-date Regulatory requirements and hassle-free ‘Ad Promo’ Submission to HA.
- Comprehensive experience in the intricacies of Life Sciences Content Marketing.