Overview
We provide end-to-end Corrective and Preventive Action (CAPA) management services tailored specifically for the medical device sector. Our solutions help organizations identify, investigate, and resolve quality issues while proactively preventing their recurrence.
With deep expertise in Regulatory compliance and industry best practices, we support your team in:
- Root cause analysis and risk assessment
- CAPA planning, implementation, and effectiveness checks
- Documentation aligned with FDA and ISO standards
- Integration with your QMS for seamless quality oversight
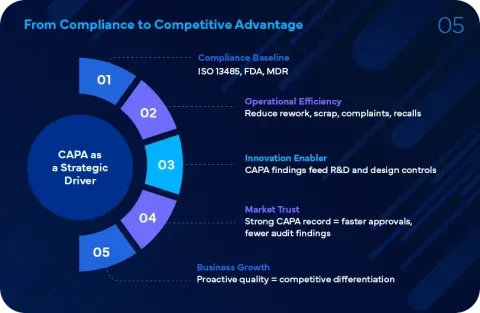
Our services are designed to help companies effectively address quality issues, prevent recurrence, and maintain compliance with global Regulatory standards such as FDA 21 CFR Part 820 and ISO 13485.
What is CAPA?
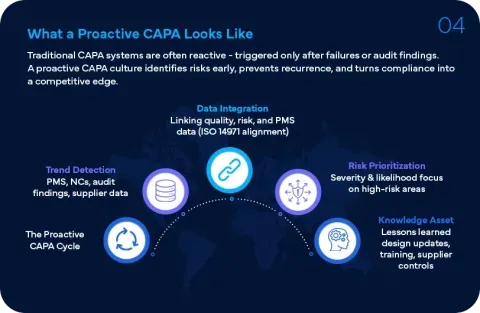
Corrective Action Preventive Action (CAPA) is an approach used to investigate and resolve quality issues along with identifying their causes. It was introduced as a result of Food and Drug Administration’s (FDA) requirement under FDA 21 CFR 820.100. The CAPA mainly consists of two functions:
Corrective Action – The goal of Corrective Action is to identify the root cause of the product and quality problems and take appropriate action against them. This includes:
- Review and definition of a problem
- Identification of the root cause of a problem
- Development of action plan for correction and prevention
- Implementation of the plan
- Evaluation of the plan efficiency

Preventive Action – The goal of Preventive Action is to prevent the problem from recurring in the near future. This includes:
- Identification of potential problems
- Identification of root cause of the problem
- Development of recurrence prevention plan
- Implementation of the plan
- Review of effectiveness of actions taken for prevention

CAPA and The Procedures
To implement an effective CAPA plan, the following steps must be followed:
- Identify potential problems related to quality, product, or non-conformity
- Evaluate the seriousness of the problem and how it would impact the business
- Evaluate the available procedures for investigation
- Analyze the problem with accurate data
- Create an action plan that addresses all the problems and solutions to prevent them
- Implement the plan
- Perform regular follow-ups to ensure the effectiveness of the solutions
By leveraging our deep expertise in CAPA and related quality management areas, medical device companies can:
- Enhance their quality systems
- Improve product safety and reliability
- Ensure consistent Regulatory compliance

For all QMS-related queries regarding our services, reach out to our experts at Freyr at sales@freyrsolutions.com