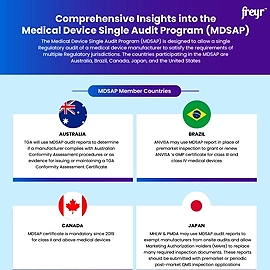
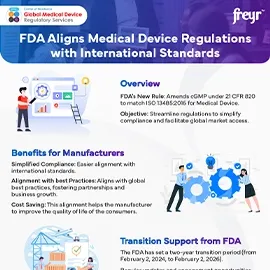
Overview
Freyr helps medical device companies manage QMS documentation efficiently while ensuring Regulatory compliance
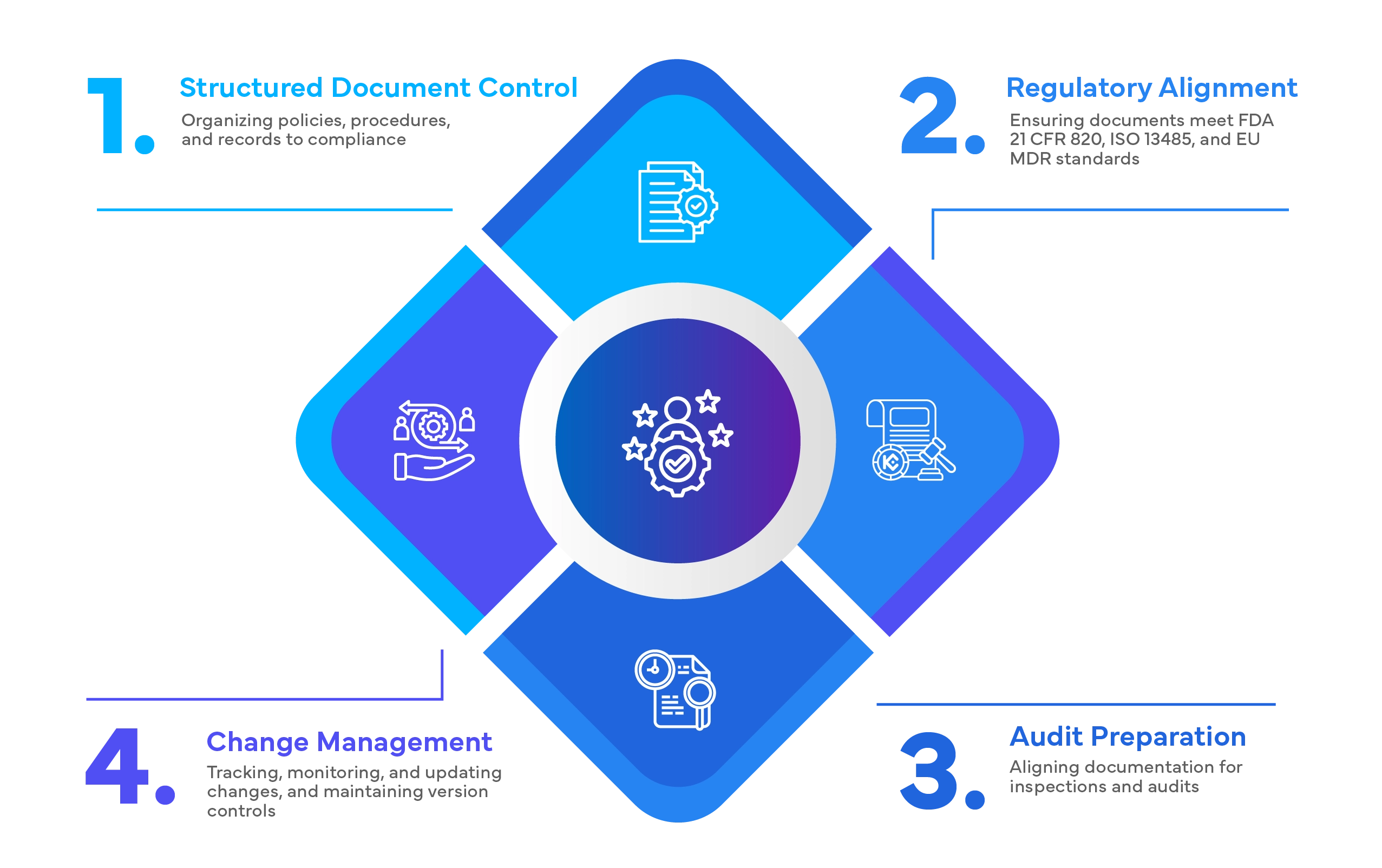
Our latest ISO 13485:2016 Quality Management System Toolkit combines the documentation templates, SOPs and expert support to streamline your workload and ensure you meet all the guidelines and regulations with ease.
Learn more about the key components of a Quality Management System (QMS) for medical devices.