End-to-End Global Product License Maintenance
Turning your breakthroughs into real world impact - Our shared mission
We navigate the regulatory maze for you with true end-to-end product compliance ownership that scales with your mission, empowering you to focus on life-changing innovation.
Our unique model consolidates consulting expertise, operational execution, and proprietary AI technology into a fully integrated KPI-driven partnership, eliminating the need for client-side regulatory investments in people, processes, and technology. Freyr doesn’t just support regulatory compliance - we own it.
Consulting
- Regulatory strategy during product development
- Product classification
- Optimal regulatory pathways for product registrations
- Gap analysis
- Systems consultation
- Centralization & harmonization of SOPs
- Process optimizations that eliminate redundancies & accelerate timelines
- Custom regulatory intelligence reports
Services
- Health Authority (HA) interactions
- Meeting packages for HA
- Preparation of initial regulatory dossiers
- HA query resolution support
- Change controls assessments
- Preparation & submission of post-approval change applications
- End-to-end product life-cycle regulatory management
Technology
AI-First, Cloud-Native Regulatory Platform
- Centralized ecosystem for managing registrations, submissions, labeling, artwork, documents, and regulatory intelligence
- Embedded business intelligence and Freya chatbot enable intuitive, conversational access to data and documents
- AI-powered insights for accelerated decision-making
- Integrated project planning & tracking, automated notifications, KPI monitoring, and workflow management
- Seamless automation and advanced content management
- Built on a GxP-compliant, enterprise-grade architecture ensuring security, scalability, and reliability
Key Differentiators: Why Freyr’s Model is Unmatched
Gain access to a globally unified regulatory model that no other provider can match - combining deep domain expertise, global reach, and Local Health Authority insights. It's a partnership built for precision, scalability, and procurement value.
- 100% On-time renewals
- 72-hour Response SLA for regulatory
inquiries - 5-year TCO reduction
(30–40% savings vs. in-house)
- 80% AI Regulatory technology
automates 80% of submissions - Real-time Global License Dashboard for
real-time market tracking - Zero Zero tech deployment costs
| Cost Factor | Traditional Model | Freyr Model |
|---|---|---|
| People | Salaries + training + turnover | Included |
| Technology | $250K+ in software/licenses | Included |
| Process | Consultants + rework costs | Included |
| Risk | Fines for missed deadlines | Freyr assumes liability |
The Freyr Partnership vs. Legacy Approaches
Simplify your vendor landscape, amplify your impact. Drive your business outcomes with a singular strategic partnership delivering what multiple vendors, platforms and systems can’t.
| DIY Compliance | Outsourced Fragmented Services | Freyr End-to-End Ownership | |
|---|---|---|---|
| Consulting | Hire internally | Separate advisory firm | Embedded experts |
| Execution | Build a team | Multiple vendors | Single accountable team |
| Technology | Costly in-house tools | Manual processes | AI platform included |
| Cost Predictability | Budget overruns | Hidden fees | Fixed per-user pricing |
| Risk | Yours alone | Shared (but diluted) | Freyr-backed KPIs |
Need Full-Spectrum Regulatory Support?
Connect with Our Experts for a Tailored Compliance Roadmap.
Other Engagement Models
Wherever You Are on the Regulatory Journey - We Meet You There
Tailored Outsourcing Models: Scaling Regulatory Partnerships
- Specialized Function Expertise
- Advanced Technology Access
- Operational Efficiency
- Cost-Effectiveness
- Flexible Support
- Local Regulatory Expertise
- Optimal Workforce Management
- Faster Market Entry & Approvals
- Local Partnerships/LR Agents
- Compliance Monitoring
- Consistent Quality Standards
- Lifecycle Scalability
- Risk Mitigation
- Cost Benefits
- Niche Product Submission Expertise
- Business Driven
- Product Compliance
- Harmonization of Sites
- Mergers and Acquisitions
- Health Authority Driven
Additional Models for Regulatory Excellence

Designed for agility, the Time & Material model enables flexible, on-demand access to regulatory expertise, ideal for projects with dynamic scope and uncertain timelines. With billing based on actual resource utilization and supported by detailed tracking and reporting, this model empowers procurement teams to retain control over budgets while scaling resources as needed-without contractual rigidity.
The Unit-Based Pricing model delivers structured, performance-driven engagement by assigning fixed costs to standardized regulatory deliverables. This outcome-focused approach enhances predictability, facilitates volume-based cost efficiencies, and enables procurement to benchmark performance with clarity-making it particularly effective for high-volume, multi-country regulatory operations.
The Fixed Price model offers complete financial predictability for projects with well-defined scope and deliverables. With an all-inclusive, pre-negotiated cost, this model eliminates cost overruns, simplifies procurement governance, and transfers delivery accountability to Freyr-ideal for short- to mid-term initiatives where stability and precision are paramount.
The FTE-Based model provides long-term, embedded regulatory support through dedicated professionals allocated on a full-time or fractional basis. Tailored for continuity and strategic alignment, this model allows for seamless integration with client teams, predictable pricing, and the flexibility to adjust resource levels in response to shifting priorities-ideal for evolving and enterprise-scale programs.
Success Stories
Procurement Decisions Define Regulatory Success
Select a partner who aligns with your vision for cost control, continuity, and compliance integrity.
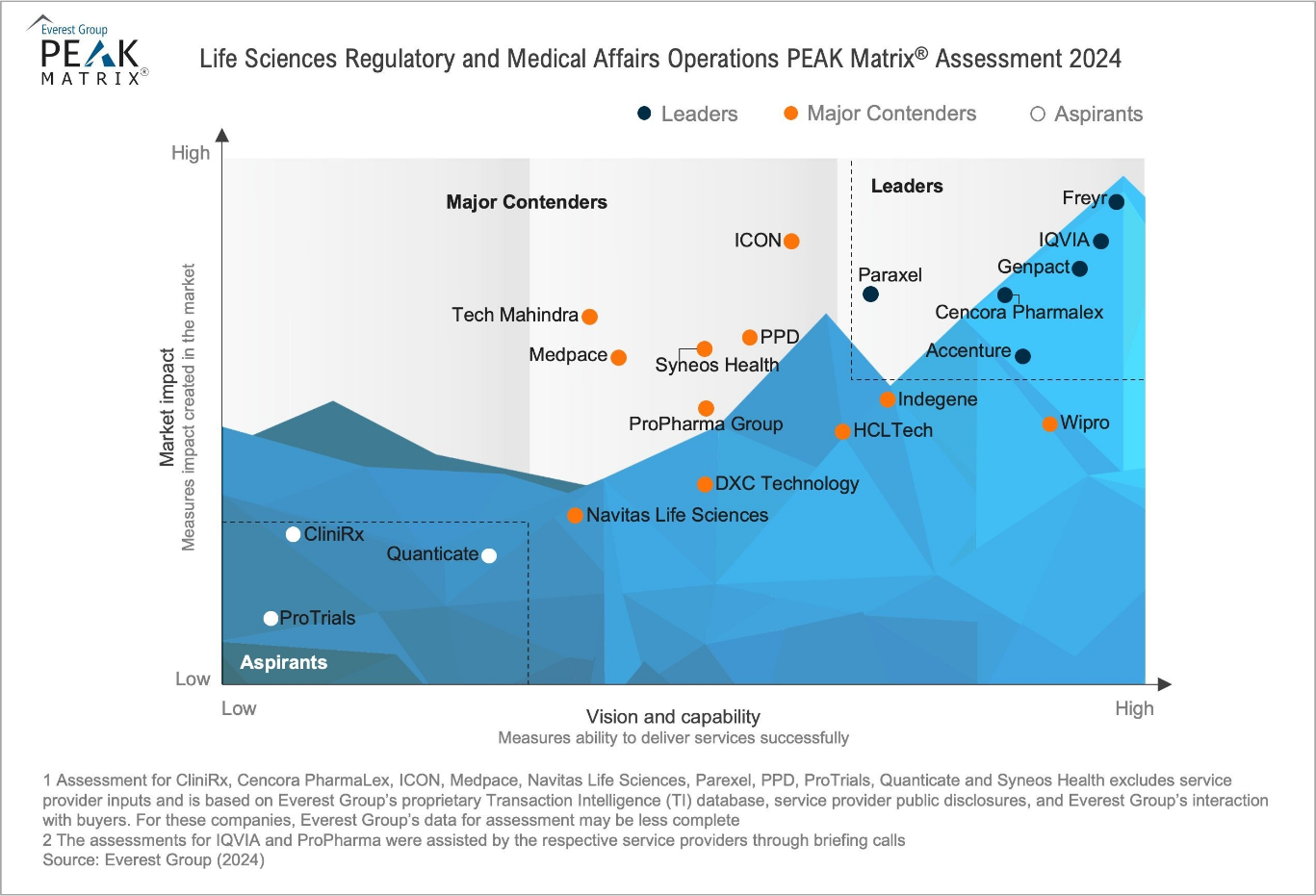
Why is Freyr Recognized as the Industry Leader?
The largest global regulatory solutions and services company in the life sciences industry
We support large, medium, and small size global life sciences companies (Pharmaceutical | Generics | Medical Device | Biotechnology | Biosimilar | Consumer Healthcare | Cosmetics) in their entire Regulatory value-chain, ranging from Regulatory Strategy, Intelligence, Dossiers, Submissions, etc., to Post-approval/Legacy Product Maintenance, Labeling, Artwork Change Management, and other related functions. Freyr is also expanding its footprint into other key areas like Pharmacovigilance.
Headquartered in New Jersey, USA, Freyr has regional offices across the UK, Germany, South Korea, Switzerland, UAE, Canada, Colombia, Mexico, Chile, Peru, Brazil, Singapore, Australia, Malaysia, South Africa, Thailand, Hong Kong, Nigeria, New Zealand, Sri Lanka, Poland, China, Japan, and a Global Delivery Center in Hyderabad, India.
![]()
Freyr Solutions Embraces the Spirit of Giving During Joy of Giving Week with CSR Initiative for Aadarana Trust Children
![]()
Sopot Beach Cleanup: A Step Toward Sustainability
![]()
Freyr UK Gives Back: A Day of Community Building
![]()
Pantry donations in the UK
![]() ISO 13485 : 2016
ISO 13485 : 2016
Provision of Global Consulting Services Applicable to the Medical Device and In Vitro Diagnostic (IVD) Industry for
![]() ISO 27001 : 2013
ISO 27001 : 2013
Information Security Management Services cover the Software and Regulatory Services of the following departments
![]() ISO 9001 : 2015
ISO 9001 : 2015
Quality Management System covers the following departments