Introduction:
Food supplements are products intended to supplement the diet and contain one or more ingredients such as vitamins, minerals, amino acids, fatty acids, fiber, or other substances with nutritional or physiological effects. Understanding the nutraceutical classification is crucial for ensuring safety and regulatory compliance across different regions.

Regional Classification of Dietary Supplements
Comparison Table: Classification Terminology by Region
| Region | Terminology | Regulatory Body | Key Regulations |
|---|---|---|---|
| United States | Dietary Supplements | FDA | DSHEA 1994, GMPs, Labeling Requirements |
| European Union | Food Supplements | EFSA | Directive 2002/46/EC, Health Claims Rules |
| United Kingdom | Food Supplements | FSA | Post-Brexit Adaptations |
| India | Health Supplements | FSSAI | Health Supplements Regulations |
| Japan | FOSHU/FFC | MHLW | Scientific Evidence, Government Approval |
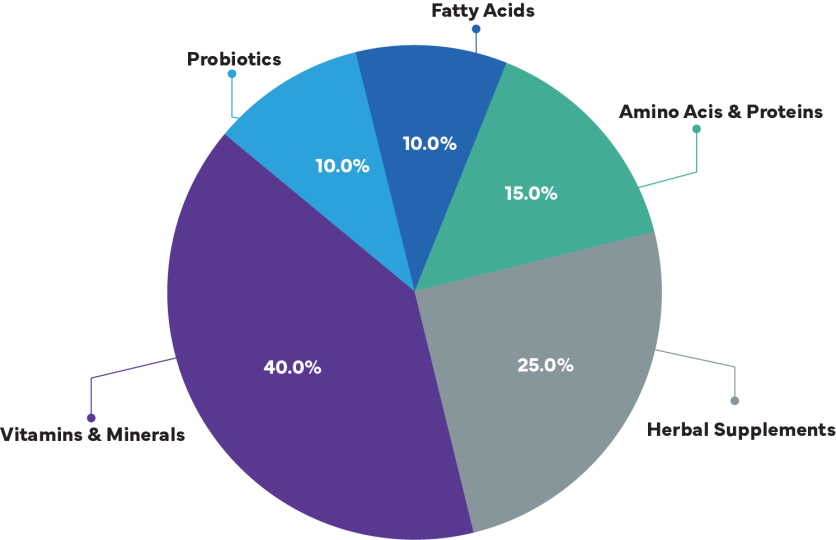
Types of Food Supplements
Pie Chart: Market Share by Supplement Type

Regulatory Considerations
Checklist: Key Regulatory Requirements
- Compliance with regional regulations
- Accurate labeling with ingredient list and recommended dosage
- Safety and efficacy data for health claims
- Adherence to Good Manufacturing Practices (GMP)
This checklist serves as a quick reference for manufacturers to ensure food regulatory compliance.
How Freyr Can Help
- Food and food supplement classification or product categorization.
- Formula review for acceptability of vitamins, minerals, and food additives.
- Region-specific food and food supplement classification.
- Regulatory pathway or strategy for deciding on the classification.
- Pre-formulation Regulatory assessment.
- Providing recommendations on the reformulation of the product.
- Identification of prohibited ingredients.


Why Choose Freyr
- Cost-effective consultation services.
- End-to-end Regulatory consultation for classification of functional foods/food products/food product classification.
- Qualified team of experts with hands-on experience across all categories of Food and Dietary Supplements (FDS) like health supplements, dietary supplements, nutraceuticals, health functional foods, health functional beverages, foods for special dietary use, and much more.
- Support for region-specific Regulatory complexities.
- Extensive partner network across the globe.
- A strong relationship with different health authorities (HAs).
- A structured approach to ensure quick market access.
Ensure accurate classification. Stay compliant, stay market ready.
Partner with Freyr for expert guidance on global Food and Food supplement regulations.