Small and medium-sized life science companies are the backbone of the Healthcare industry, but Regulatory hurdles often hamper their potential for ground-breaking innovation. These companies face an uphill battle to bring their products to markets, with limited resources and expertise in navigating the complex Regulatory landscape. However, by adopting effective strategies and seeking expert guidance, they can overcome the odds and emerge as leaders in their field. With the right approach, Regulatory submissions can become an opportunity for growth and success rather than a roadblock to innovation.
Here are some of the Regulatory submission challenges faced by small and midsize organizations:
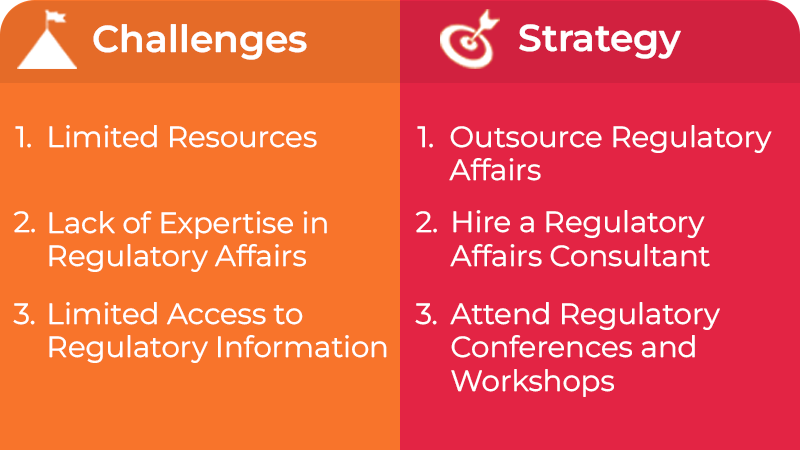
Challenge 1: Limited Resources
Small and medium-sized life science companies often need more resources, which can make Regulatory submissions more difficult. These companies may need more money to hire a full-time Regulatory affairs team and access to the latest technology and resources to manage the submission process.
Strategy: Outsource Regulatory Affairs
One strategy for such companies is outsourcing their Regulatory affairs to a specialized consulting firm. These firms have the expertise and resources needed to manage the submission process and can guide on Regulatory requirements, submission timelines, and best practices for preparing a successful submission. By outsourcing Regulatory affairs, companies can free up internal resources and focus on other critical aspects of their business.
Challenge 2: Lack of Expertise in Regulatory Affairs
Small and medium-sized life science companies may need to gain in-house expertise in Regulatory affairs.
Strategy: Hire a Regulatory Affairs Consultant
Another strategy for small and medium-sized life science companies is to hire a Regulatory affairs consultant. With the expertise needed to guide companies through the Regulatory process, a Regulatory affairs consultant can ensure that the submissions are accurate and complete and can avoid potential delays or rejections.
Challenge 3: Limited Access to Regulatory Information
The companies have limited access to the latest Regulatory information, making it difficult to stay current on changing regulations and requirements.
Strategy: Attend Regulatory Conferences and Workshops
The companies can attend Regulatory conferences and workshops, which provide opportunities to learn about the latest Regulatory developments and requirements and to connect with Regulatory experts and other industry professionals. These events can help small and midsize life sciences companies to stay up-to-date with the changing regulations and requirements and ensure that their submissions comply with current standards.
CHALLENGE STRATEGY
In conclusion, small and medium-sized life science companies face unique challenges regarding w.r.to Regulatory submissions. However, by outsourcing Regulatory affairs, hiring a Regulatory consultant, and attending Regulatory conferences and workshops, they can overcome the challenges and successfully navigate the Regulatory landscape. By doing so, they can ensure that their products are brought to market quickly and efficiently while maintaining the highest safety and efficacy standards.
Freyr SUBMIT PRO offers an effective and cost-efficient solution, a pay-per-use model based on usage volume, which allows users to minimize their investment while still accessing secure and certified cloud solutions. With over a decade of experience, we offer Freyr SUBMIT PRO, a comprehensive eCTD tool that offers competent, timely submissions while maintaining high safety and efficacy standards. So, if you're looking to save on Regulatory submission costs and ensure smooth and seamless submissions, consider using Freyr SUBMIT PRO and #Dosmartsubmissions.
Would you like to learn more? Request a demo.