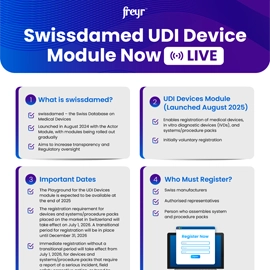
Overview and Definition
Medical device Mergers and acquisitions (M&A) in the medical device industry involve complex Regulatory challenges across multiple jurisdictions. Integrating two companies requires harmonizing Quality Management Systems (QMS), managing Regulatory submissions, and aligning clinical and post-market processes.
Freyr’s specialized medical device Mergers and acquisitions Regulatory services for medical devices provide end-to-end support to help your organization navigate these challenges seamlessly, ensuring continued compliance with ISO 13485, MDR, FDA regulations, and other global standards.
Medical Device M&A Compliance Process
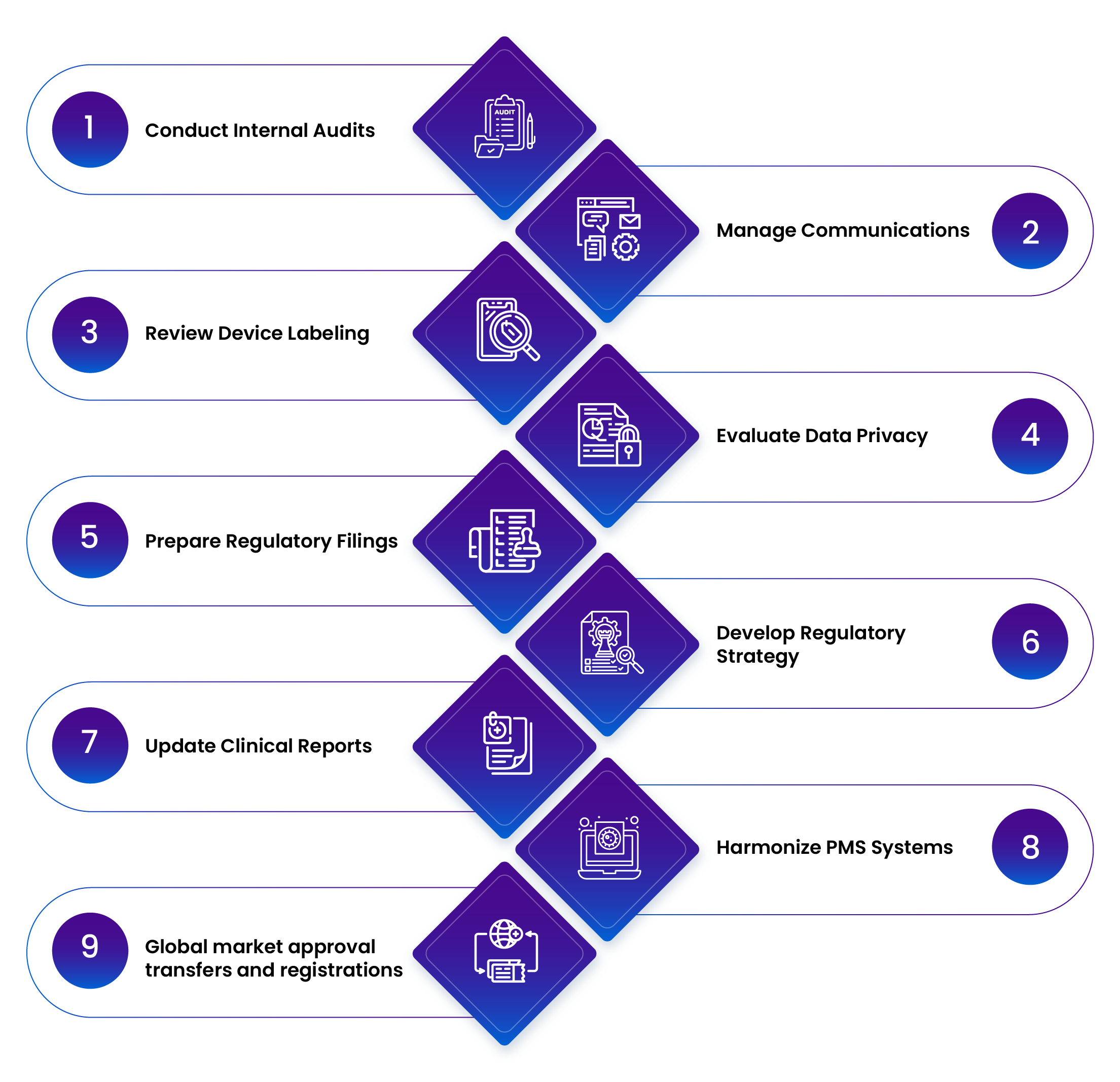
Freyr Services for Mergers and Acquisitions – Current Portfolio
Regulatory Due Diligence
- Comprehensive assessment of the target company's regulatory compliance, including QMS, product registrations, clinical data, and labeling.
- Identification of potential regulatory risks and liabilities.
- Gap analysis and remediation planning.
Regulatory Strategy & Planning
- Developing a comprehensive regulatory strategy for integrating the two companies' product portfolios.
- Planning for the transfer and updating of regulatory registrations and licenses across all relevant markets.
- Developing a timeline for regulatory activities.
QMS & CAPA Integration
- Harmonizing the QMS of the two companies to ensure compliance with ISO 13485 and other relevant standards.
- Developing and implementing a unified QMS documentation system.
- Conducting internal audits and gap analyses.
- Regulatory Submissions & Registrations:
- Preparing and submitting all necessary Regulatory filings for the transfer and updating of product registrations.
- Managing communications with Regulatory authorities.
Data Privacy & Cybersecurity Integration
- Conducting a comprehensive assessment of data privacy and cybersecurity risks.
- Developing and implementing a plan for integrating data privacy and cybersecurity systems.
- Ensuring compliance with GDPR and other relevant regulations.
- Data mapping of all data that is transfered.
Technical File Updates
- Clinical Data & Evaluation Management:
- Assessing the validity and transferability of clinical data.
- Updating clinical evaluation reports (CERs) as needed.
- Managing post-market clinical follow-up (PMCF) activities.
Labeling Compliance
- Reviewing and updating labeling materials to reflect the new ownership and ensure compliance with local regulations.
- Creating a unified labeling strategy.
PMS Integration
- Combining the PMS systems of the two companies.
- Harmonizing adverse event reporting and trend analysis processes.
Global Registrations & Transfers
- Transfer product registrations across global markets
- Coordinate with Regulatory authorities for approvals
- Maintain continuity of market access during transition
Freyr’s Services
PMS INTEGRATION
- Combining the PMS systems of the two companies.
- Harmonizing adverse event reporting and trend analysis processes.
REGULATORY DUE DILIGENCE
- Comprehensive assessment of the target company's regulatory compliance, including QMS, product registrations, clinical data, and labeling.
- Identification of potential regulatory risks and liabilities.
- Gap analysis and remediation planning.
REGULATORY STRATEGY & PLANNING
- Developing a comprehensive regulatory strategy for integrating the two companies' product portfolios.
- Planning for the transfer and updating of regulatory registrations and licenses across all relevant markets.
- Developing a timeline for regulatory activities.
QMS & CAPA INTEGRATION
- Harmonizing the QMS of the two companies to ensure compliance with ISO 13485 and other relevant standards.
- Developing and implementing a unified QMS documentation system.
- Conducting internal audits and gap analyses.
- Regulatory Submissions & Registrations:
- Preparing and submitting all necessary Regulatory filings for the transfer and updating of product registrations.
- Managing communications with Regulatory authorities.
DATA PRIVACY & CYBERSECURITY INTEGRATION
- Conducting a comprehensive assessment of data privacy and cybersecurity risks.
- Developing and implementing a plan for integrating data privacy and cybersecurity systems.
- Ensuring compliance with GDPR and other relevant regulations.
- Data mapping of all data that is transfered.
TECHNICAL FILE UPDATES
- Clinical Data & Evaluation Management:
- Assessing the validity and transferability of clinical data.
- Updating clinical evaluation reports (CERs) as needed.
- Managing post-market clinical follow-up (PMCF) activities.
LABELING COMPLIANCE
- Reviewing and updating labeling materials to reflect the new ownership and ensure compliance with local regulations.
- Creating a unified labeling strategy.
Industry Challenges and Freyr’s Solutions
Working as your dedicated merger and acquisition consultant for medical device Regulatory affairs, we address the most common challenges:

Challenge
Variability in QMS Maturity and Compliance Status

Freyr’s Service & How We Support
- Quality Management System Harmonization Services: Our Merger and Acquisition consultant team conducts gap analyses and internal audits to unify and align Quality Management Systems (QMS) with ISO 13485 and Regulatory standards for seamless integration.
Complex Regulatory Submission and Registration Transfers
- Regulatory Affairs SoMAD ( Spin-off, Medical device Mergers and acquisitions, Divestiture ) Services: Manage Regulatory submissions, license transfers, and communications with global authorities to ensure timely approvals and ongoing compliance.
Ensuring Labeling Compliance Reflecting New Ownership
- Labeling Update and Compliance Services: Develop and implement unified, compliant labeling strategies to reflect new ownership and meet all regional Regulatory requirements.
Managing Clinical Evaluation Data and PMCF Obligations
- Clinical Data and Evaluation Management: Assess clinical data transferability, update Clinical Evaluation Reports (CERs), and manage Post-Market Clinical Follow-up (PMCF) activities.
Addressing Data Privacy and Cybersecurity Compliance
- Data Privacy and Cybersecurity Assessments: Evaluate and integrate data privacy and cybersecurity systems to ensure compliance with GDPR and other global regulations.
Combining Post-Market Surveillance Systems and Reporting
- Pharmacovigilance and Medical Affairs SoMAD Services: Harmonize post-market surveillance systems, adverse event reporting, and trend analysis for consistent and compliant safety monitoring.
We Do NOT Provide
Legal Medical device Mergers and acquisitions advice
Financial or transactional consulting
Manufacturing or operational integration services
Note: While we serve as your Regulatory merger and acquisition consultant, we focus exclusively on Regulatory compliance and do not provide legal, financial, or operational M&A services
Why Partner with Freyr?

Case study
End-to-end QMS Implementation for SaMD
A US-based Life Sciences Consulting Firm partnered with Freyr for end-to-end QMS implementation for their SaMD. Freyr developed a risk management plan, trained staff on SOPs, and ensured ISO 13485-compliant execution.