Foreign Manufacturing Registration for Medical Devices in Japan Overview
Japan’s Pharmaceuticals and Medical Devices Act (PMD Act) mandates all foreign manufacturers to register their relevant manufacturing facilities through the Foreign Manufacturer Registration (FMR/TOUROKU) process.
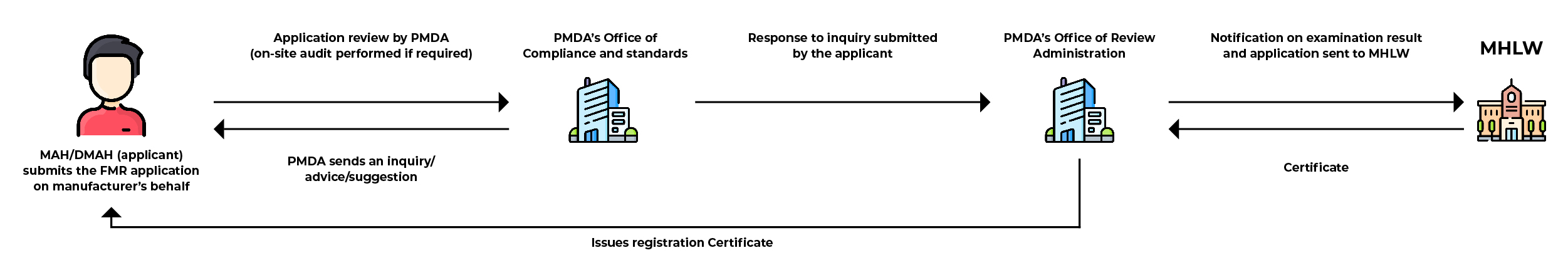
FMR Review Process
The FMR certificate is valid for five (05) years. The Ministry of Health, Labour, and Welfare (MHLW) of Japan advises starting the renewal procedure at least five (05) months before your certificate expires.
At Freyr, we specialize in providing meticulous medical device documentation services designed to ensure Regulatory compliance and streamline your product development processes. With our expertise and attention to detail, we facilitate a seamless journey from concept to market.
Frequently Asked Questions (FAQs)
All foreign manufacturers willing to export medical devices to Japan must register their manufacturing facilities with the Ministry of Health, Labor, and Welfare (MHLW). This registration procedure is called Foreign Manufacturer Registration (FMR), which formerly was known as “Foreign Manufacturer Accreditation (FMA)” or “Accreditation of Foreign Manufacturers (AFM).”
To ensure that the foreign manufacturer is qualified to take part in the product registration procedure, manufacturing facilities are evaluated. The following sites need to be registered for each medical device product:
- Design Facility - The location where the product is developed and development records are kept.
- Main Assembling Plant - The facility that executes assembly processes; this facility is largely accountable for ensuring that the QMS requirements are followed or for the manufacturing of products.
- Sterilizer - The location where the sterilizing process is carried out (for sterile medical device products).
- Domestic Distribution Center in Japan - The location that handles the product's final distribution to the Japanese market and stores it.
Manufacturers must ensure these pre-requisites are fulfilled before the FMR application is submitted. All the documents must be in Japanese language only.
- Appointing MAH/DMAH – All foreign manufacturers must appoint an MAH/DMAH.
- Business Number Registration- Obtain a business number for each manufacturing facility.
- "Shomeisho“- Self-declaration of Medical Condition ("Shomeisho") of the senior manager representing the manufacturer.
- Facility Map - Drawings, floor plans, pictures, etc. of the building(s) in scope.
Medical Device Regulatory Consulting – Proven Expertise
Foreign Manufacturing Registration for Medical Devices in Japan