Pharma Regulatory Services in Chile – Overview
Chile's pharmaceutical market is regulated by the Instituto de Salud Pública de Chile (ISP), which oversees the authorization, registration, and surveillance of medicines. All pharmaceutical products require registration prior to sale, ensuring they meet the quality, safety, and efficacy standards set by international guidelines.
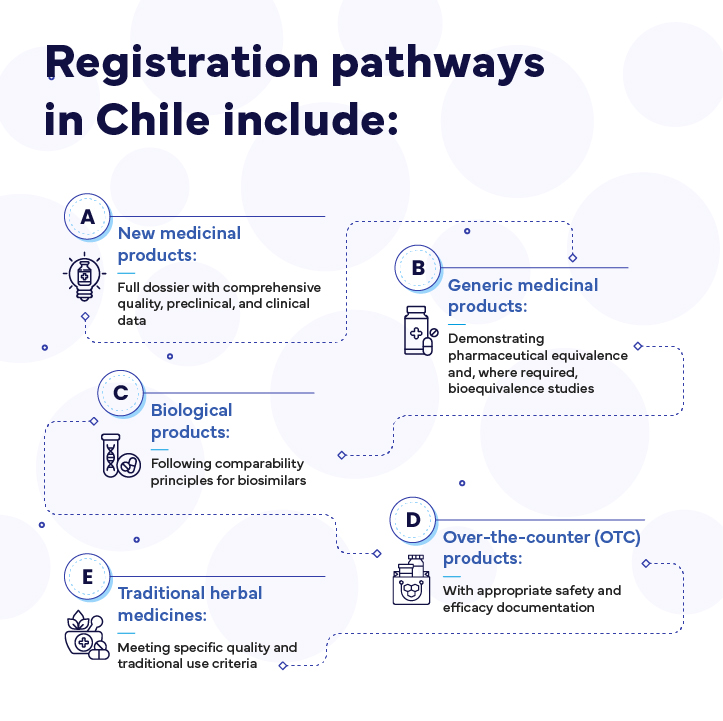
Registration pathways in Chile include:
Key requirements include Spanish documentation, apostilled certificates from the origin, a registered holder in Chile, and GMP compliance certificates. Bioequivalence studies are required for specific active ingredients as designated by the ISP.
The Regulatory landscape demands expertise in local submission processes, documentation, and compliance. Foreign manufacturers face complex administrative tasks while ensuring adherence to Chilean labeling, pharmacovigilance, and quality standards.
Freyr provides comprehensive Regulatory support for pharmaceutical companies seeking market authorization in Chile, from strategic planning through post-approval lifecycle management.
Pharma Regulatory Services in Chile - Freyr Expertise
Registration Strategy & Dossier Preparation
- CTD-format dossier authoring for ISP submissions
- Pathway assessment/s (innovator, generic, biosimilar)
- Regulatory Intelligence (RI) and Gap Analysis
- Evidence planning for quality, preclinical, and clinical modules
Local Representation Services
- Chilean-domiciled Registration Holder services
- ISP liaison and official correspondence management
- Manufacturing site registration and compliance services
Documentation & Administrative Support
- Certificate of Pharmaceutical Product (CPP) management
- GMP certificate compilation and apostille coordination
- Spanish translation and notarization of Regulatory documents
- Power of Attorney preparation and legalization
Bioequivalence & Quality Services
- Bioequivalence study planning and oversight
- Biowaiver justification packages preparation
- Analytical method validation support
- Stability protocol development and data evaluation
Submission & Lifecycle Management
- Electronic submission via ISP platforms
- Query response and deficiency management
- Post-approval variations (CMC, Labeling, Administrative, etc.) and amendments
- Annual maintenance and renewal support
Compliance & Pharmacovigilance
- Chilean labeling and Artwork review
- Pharmacovigilance system implementation
- Adverse Event (AE) reporting procedures
- Risk Management Plan (RMP) development
Our Location in Chile
Av Manquehue Nte 151,
Of. 1205, Las Condes,
Chile
