
In the ever-changing and highly regulated medical device manufacturing field, guaranteeing product quality and patient safety is not just about a legal obligation—but also a strategic imperative. Effective complaint management and vigilance systems, as per ISO 13485:2016, form the backbone of any robust Quality Management System (QMS). These mechanisms ensure compliance with global regulations and enhance customer trust and brand credibility.
Let’s dive into how complaint handling and vigilance play a fundamental role in sustaining quality and regulatory compliance in the medical device sector, with insights aligned to ISO 13485:2016 and supported by global Regulatory frameworks.
Understanding Complaint Management Under ISO 13485:2016
ISO 13485:2016 defines a complaint as any written, oral, or electronic communication alleging faults related to a medical device's identity, quality, durability, reliability, usability, safety, or performance. This standard requires medical device companies to establish documented procedures for complaint handling, including evaluating, investigating, and resolving complaints as a part of effective quality management system.
A complaint-handling system is key to compliance, maintaining customer satisfaction, and improving device quality. The process has to be organized and observable, involving the following steps:
- Complaint Receipt and Documentation
All complaints must be logged without delay, with as much detail as possible regarding the nature of the issue, device specification, and the model, their usage conditions, and any consequences experienced by the user or patient. - Evaluation for Reportability
Once they are recorded, complaints must be evaluated to determine if they are reportable incidents. For instance, in the U.S., medical device manufacturers are required to report certain device problems under the Medical Device Reporting (MDR) system regulated by the FDA. The applicable regulations for reporting timelines shall be followed by the manufacturers. - Investigation and Root Cause Analysis
A detailed investigation should follow for valid complaints. Identifying the root cause is essential for implementing effective corrective and preventive actions (CAPA). - Corrective and Preventive Actions (CAPA)
Based on the medical device findings, companies must initiate CAPAs to address both the immediate issue & recurrences and prevent future occurrences. - Feedback Loop and Closure
Once the issue is resolved, the complaint should be formally closed with complete documentation and communication back to the complainant, if applicable for the medical device.
Vigilance: A Core Pillar of Post-Market Surveillance
Vigilance refers to the monitoring and reporting of opposing events and incidents once a medical device is on the market. Under ISO 13485:2016 and corresponding global regulations, this ongoing surveillance is essential for the early detection of potential risks and the implementation of timely interventions.
The European Union’s vigilance system, as updated under the Medical Device Regulation (EU MDR), mandates medical device manufacturers to have post-market surveillance plans. These plans must include processes for collecting and examining data from a device’s real-world usage.
Similarly, in India, the Central Drugs Standard Control Organization (CDSCO) governs the vigilance and complaint processes for medical devices. It encourage reporting of adverse events and product failures to continually enhance medical device safety and efficacy.
Key elements of a strong vigilance system include:
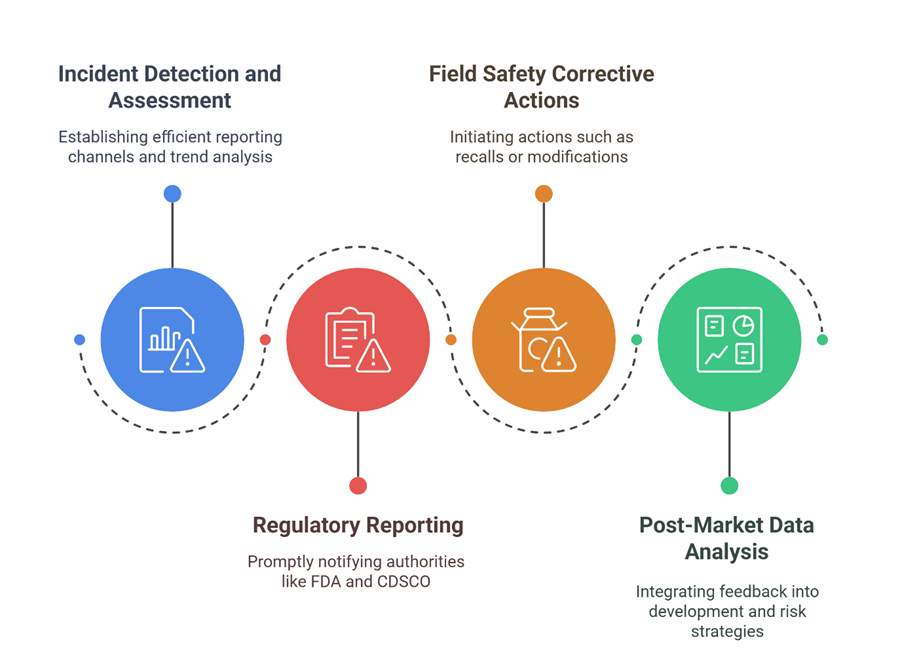
Building an Integrated Complaint and Vigilance Strategy
Medical device manufacturers can elevate their QMS by integrating complaint handling and vigilance into a single cohesive system. Here are best practices:
Clear SOPs and Role Definitions
Standard Operating Procedures (SOPs) should outline the steps for complaint intake, evaluation, reporting, and resolution. It defines who is responsible at each stage to avoid delays and gaps.
- Employee Training and Awareness
Regular employee training ensures that staff understand how to identify and handle complaints and adverse events effectively. - Digital Tools and Automation
Leveraging QMS software can streamline complaint tracking, documentation, and reporting. Automation enhances traceability and conformity to ISO 13485:2016. - Quality Culture and Continuous Improvement
Promoting a philosophy where feedback is valued encourages proactive issue reporting and fosters innovation in quality improvements.
Compliance and Beyond: A Marketing Advantage
Implementing ISO 13485:2016 compliant systems for complaint management and vigilance not only ensures Regulatory observance but also positions a company as trustworthy and quality-focused. In an industry where safety and performance directly impact lives, such commitment can be a powerful marketing tool.
As per FDA , aligning with international standards improves access to global markets, strengthens customer confidence, and reduces the risk of costly recalls or litigation.
| Region | Regulatory Framework | Reference Clause No/ Article for Complaint Handling | Reference Clause No/ Article for Vigilance / Adverse Event Reporting |
| European Union | EU MDR 2017/745 | Article 83, Annex III (PMS system); Article 10(9) | Articles 87–92 (Vigilance System), Annex III |
| United States (FDA) | 21 CFR Part 820 (QSR) | §820.198 – Complaint files | 21 CFR Part 803 – Medical Device Reporting (MDR) |
| India (CDSCO) | Medical Device Rules, 2017 (MDR) | Chapter VII, Rule 25 & 26 | Chapter VII, Rule 27 |
| Global (ISO Standard) | ISO 13485:2016 | Clause 8.2.2 – Complaint Handling | Clause 8.2.3 – Reporting to Regulatory Authorities |
Conclusion
In today’s competitive medical device Regulatory landscape, effective complaint management and vigilance systems are non-negotiable. Aligned with ISO 13485:2016, these processes not only protect patients but also build a resilient, reputation-driven brand. By investing in systematic procedures, ongoing training, and post-market vigilance, manufacturers can transform Regulatory requirements into opportunities for growth and market leadership.









