
Unmet Medical Need (UMN) is one of the most pressing priorities of health systems. Constantly evolving science and technology around healthcare has harbored the potential to explore various therapeutic options. Health Authorities encourage innovative therapies that promise significant benefits to patients' quality of life. Building upon prioritizing innovation and targeting unmet patient needs for drug development, the European Medicines Agency (EMA) introduced the PRIority MEdicines (PRIME) pathway in 2016. Since then, the PRIME pathway has been a method for granting approvals for innovative therapies on an accelerated timeline. This type of facilitated pathway can be explored by any company, ranging from start-ups to mid-sized biotech to large multinational organizations. However, qualification for the pathway is limited to products under development and yet to apply for a marketing authorization through the centralized procedure. The qualifying criteria are applied rigorously. Between March 2016 and April 2022, only 24% of the applications received the endorsement, whereas 72% of PRIME applications were rejected. Of the 24% that were selected for the PRIME pathway, the majority belonged to the oncology therapeutic area.
Innovators pursuing market authorizations of rare diseases or orphan drugs may often have limited datasets available by way of evidence to support their novel drug applications, as required by regulators. Applications for Advanced Therapy Medicinal Products (ATMPs) and orphan drugs usually face such challenges. Due to limited patient population data, constant interaction with regulators is encouraged to enable better insights for scientific review and approval requirements. The PRIME pathway can be useful for such therapies due to the early involvement of regulators in providing proactive support and guidance for data gathering and benefit-risk assessment.
Merits of Following the PRIME-Pathway
- Helps innovators to develop a well-drafted development plan.
- Benefits innovators by engaging the Health Authorities at the early development stage; this contributes to drafting high-quality market authorization applications.
- Speeds up overall evaluation by reducing the average evaluation time so that medicines can reach patients more quickly.
- Supports innovators to focus their attention on the development of medication to improve patient outcomes and address unmet patient needs.
Evaluation Time in Detail
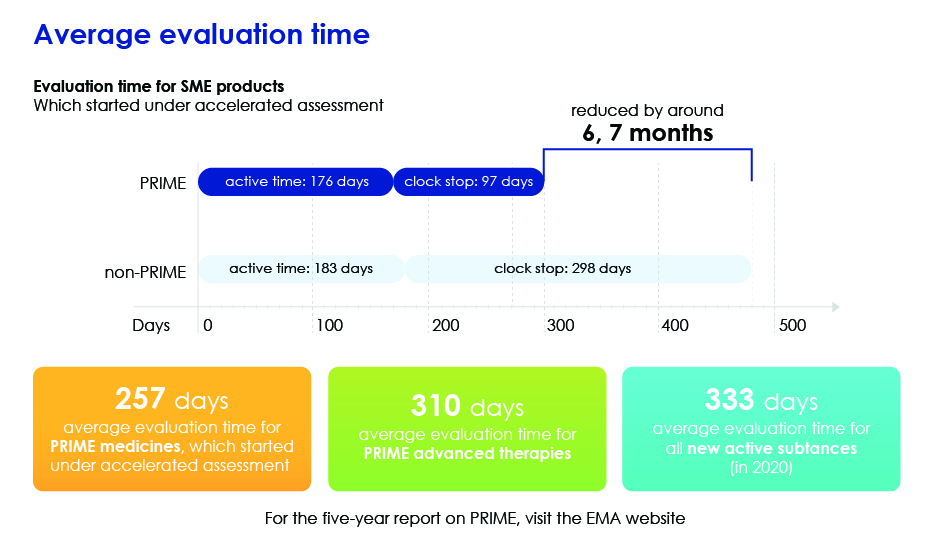
Applications for PRIME Pathway
The PRIME pathway prioritizes unmet medical needs. Out of 384 PRIME requests received since March 2016, only 18 made it all the way through to marketing authorization by June 2021.
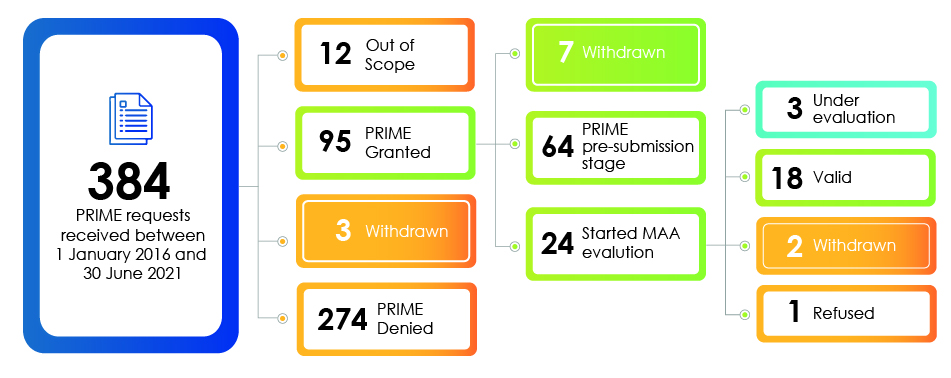
Are you PRIME-ready?
The existence of the PRIME pathway is due to significant unmet medical needs and the focus on bridging the gaps by the European Medicines Regulatory Network (EMRN). To evaluate a product’s suitability for the PRIME pathway, one must consider the following:
- Findings: Applicants must state the unmet medical need and provide a convincing argument on how their product will address the issue.
- Potential: Applicants must demonstrate the beneficial potential of the therapy in the unmet medical need criteria.
- Pre-clinical data: Applicants must present some data on the efficacy or performance of the therapy in the human biological system. Non-clinical models, however accurate, do not provide an exact overview of its behavior in the human biological system.
- Stage of development: Applicant must identify the right stage to enter the PRIME pathway to gain optimum guidance from the regulators and proceed further with the application.
Post the PRIME Grant
Once an applicant has been granted the opportunity to enter the facilitated route, the EMA will:
- Assign a rapporteur from the Committee for Medicinal Products for Human Use (CHMP) or the Committee on Advanced Therapies (CAT) in the case of advanced therapies.
- Organize a kick-off meeting with the CHMP/CAT rapporteur and a multidisciplinary group of experts to provide guidance on the overall development plan and Regulatory strategy.
- Assign a single point of contact for the applicant.
- Provide scientific advice at every key development milestone, involving an additional set of stakeholders such as health-technology-assessment bodies – to fast track access for patients to the new medicine.
- Confirm potential for accelerated assessment at the time of an application while considering marketing authorization.
Is your application for the PRIority MEdicine pathway ready (PRIME-ready)? Contact Freyr today and evaluate. Learn more about how we can help with your Regulatory strategy and PRIME pathway designation. Stay informed. Stay compliant.









