
REALIZING IDMP COMPLIANCE DEADLINE BY JULY 2016: NO TRIVIAL UNDERTAKING
There are many questions on IDMP e.g; where is the data in an organization located? Is the cleanup and alignment of current data required? How do companies organize this data for easy retrieval and submission? Does the organization have processes that can benefit from a central repository?
Achieving Identification of Medicinal Products (IDMP) compliance is no trivial undertaking, the activity must take into consideration various regulatory and operational pressures. Organizations must wait until the authorities issue final guidance and approve specifications. At the same time, waiting for these guidance’s might not leave them with sufficient time to get their affairs in order.
IDMP is a complex standard with overarching data implications requiring collaboration and co-operation among many cross-functional units. The transition gives an opportunity for an end-to-end look at an organization’s business processes and IT capabilities across many functional units. It also helps in establishing a robust change management system in place.
Organizations must understand that the good information architecture requires effort and time to accomplish and must also recognize that IDMP is not just a bigger XEVMPD. It is also important to note that ICH guidance is only part of the story, and availability of regional guidance’s is critical. One must also take into perspective that parallel regional implementations will have differing scopes and different timescales with vast breadth of data contributors.
Furthermore, Pharmaceutical companies intending to market in regulated regions must become IDMP compliant starting in 2016, recently EMA hosted IDMP Information day and shared their IDMP High level Implementation Status and Timelines. After discussion with Pharma industry, software vendors and analyzing their own system and resources availability, EMA has planned to divide the overall EU-IDMP implementation in multiple iterations. This plan will be proposed to European Commission (EC) for approval and if they are able to convince EC, then IDMP implementation in EU will be spread between 2016 to 2018. In worst case if, EC does not agree, there is no plan B. Non-compliance fines amount up to 5% of an organizations revenue; not having a good solution for initial and on-going IDMP Compliance is a risk no pharmaceutical company can afford to take.
IDMP: DEVELOPED AS GLOBAL SET OF STANDARDS UNDER ISO
In the event of any legislation which must be introduced on the identification of medicinal products in any of the ISO countries, it will be done based on ISO IDMP standards. Once different regions adopt the IDMP standards data entry will be consistent and in turn global companies and regulators will have access to cross check data for consistency across regions. Furthermore, common controlled vocabularies will strongly facilitate this process.
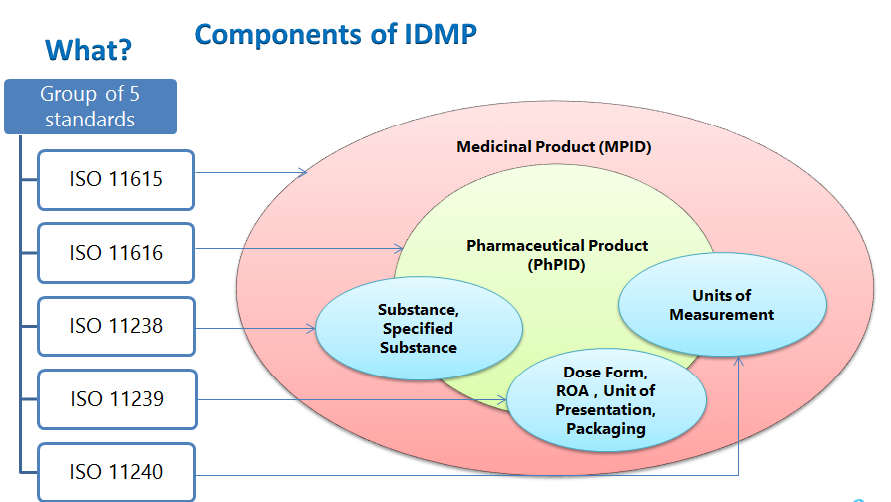
Structural elements of IDMP include medicinal product ID, pharmaceutical product ID, substance ID, controlled vocabularies-dosage form, route of administration, packaging components, units of presentations, units of measurement and the description of manufacturing process to be submitted.
IDMP STANDARDS
- ISO 11615 – Medicinal product information
- ISO 11616 – Pharmaceutical product information
- ISO 11238 – Substances
- ISO 11239 – Pharmaceutical dose forms, Units of presentation, Routes of administration and Packaging
- ISO 11240 – Units of measurement
CURRENT STATUS
ICH is preparing implementation guides and revising ISO standards
EU REGULATORS PREPARE FOR IDMP
- EMA has initiated work to liaise with the EU regulatory network to define the business cases for the IDMP data
- EMA is setting up an “EU ISO IDMP task force” recruiting experts from EMA Committees and from the EU Network Data Board to achieve this. Specifically it will:
- Define mandatory and optional ISO IDMP data elements
- Define business rules for optional data elements
- Define conformance and data types
- Define the EU governance models
US REGULATORS’ POSITION
- Active leadership in moving IDMP forward with the global (former ICH) regulators and with ISO
OTHER REGULATORS
- Switzerland – Intends to implement after EU (fast follower)
- Japan and Canada have nominated regulators to be experts on the Substance IG group
TIMELINES
EUROPEAN IMPLEMENTATION GUIDES
- Implementation guides draft initiated and guides will be available from Q1 2016
OTHERS
- FDA no date yet but intends to evolve SPL as needed
- Japan – Uncertain in ICH but now working within the Regulator’s group and ISO
- Canada – Is expected to implement but no specifics yet
- Switzerland – No updates yet
UNDERSTANDING IDMP COMPLIANCE REQUIREMENTS
IDMP requires information about medicinal products in terms of a set of standard identifiers, which are built on an identification hierarchy created while building EudraVigilance Medicinal Product Dictionary (EVMPD) or in its extended form (xEVMPD). There will be an overlap of information with those filed in Structured Product Labeling (SPL) filings in the US and other product registries globally.
However, IDMP has new identifiers, new categories and new ways to express the relationships between elements in data model. IDMP must be integrated into the organization’s DNA as it needs to drive the construction of data models throughout your enterprise. An organization’s IT infrastructure can then recognize it across multiple systems, business processes and functional units like RA, Safety, R&D, documentation and manufacturing processes.
IDMP COMPLIANCE CHALLENGES
Organizational
- Data distributed among multiple departments
- Senior sponsorship required to encourage participation
Technical
- Discover, collect and consolidate, clean data
- 250 to 300 fields per product
Co-ordination
- Management of continuous change
- Co-ordination between multiple teams
- Maintaining data regulation with internal process
IN CONCLUSION:
IDMP: PHARMACEUTICAL INDUSTRY IMPACT
Implementation of IDMP standards is expected to impact the preparation and planning of submissions and maintenance of company wide data including manufacturing data and structured substance information to registration information.
IDMP STANDARD: EFFICIENCY GAINS ENSURED UPON IMPLEMENTATION
Ensuring that your organization is ready to face the IDMP challenge will require close cooperation between multiple departments within your organization. A competent service provider with an exclusive regulatory competency portfolio, can help in moving towards IDMP compliance which can help an organization to respond to new evolving opportunities in the market place.









