
Malaysia is one of the leading markets in the cosmetics and personal care products industry. In 2019, the Malaysian cosmetics market was valued at USD 1.6 billion with a CAGR of 4.2%. According to the industry experts, the unprecedented growth of the industry is majorly a result of increasing population and employment levels, which are driving the consumers to be more conscious about the cosmetic products they use. To take part in the growing market, it is not only important for manufacturers to align with regional consumer’s preferences, but also to ensure their products are compliant enough to make a successful market-entry.
How do you plan to register your cosmetic product in Malaysia? As per the National Pharmaceutical Regulatory Agency (NPRA), the registration of cosmetic products in Malaysia is controlled by notification procedures.
Cosmetic Product Notification Procedure
To sell, supply, import or process a cosmetic product in Malaysia, a Cosmetic Notification Holder (CNH) is required to notify the product to the Director of Pharmaceutical Services (DPS) through an online portal, before placing it on the market. The major responsibilities of a CNH include:
- To carry out all the transactions with the NPRA
- To ensure that the cosmetic product meets all the requirements of the regulations and the guidelines
- To ensure that the Product Information File (PIF) is available on request with all the updated information about the safety and efficacy of the product
- In case the NPRA recalls a product from the market, the CNH must ensure that the sale of all the products is discontinued from the market
- Any changes made to the product post-marketing must be submitted to the health authority
Notification and the Submission
To submit a cosmetic product notification, CNHs must login through NPRA’s Quest System via the agency’s website. The applicants must follow the following steps to ensure successful submission:
- Registering for Quest Membership
To notify a product, the CNH must register for a quest membership - Notification of Cosmetic Product
CNH must duly fill and submit the notification form for all the products and its variants to be placed in the market
As defined by the NPRA, the registration procedure may look like:
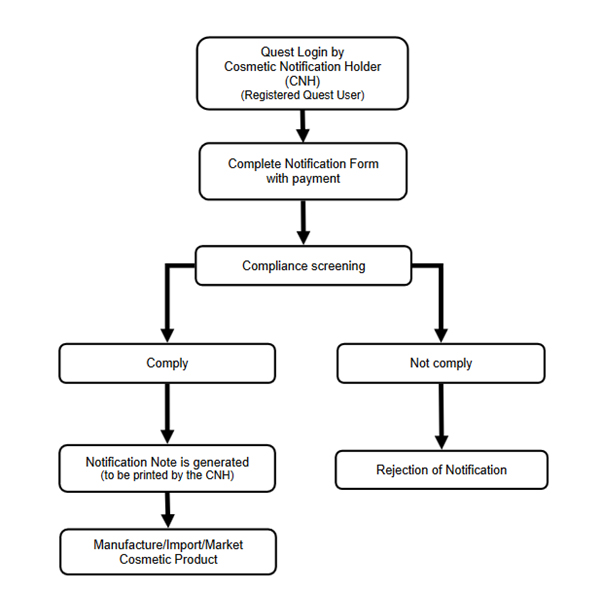
Source: NPRA
Notification Validity
The notification for any cosmetic product is valid for two years. Once the notification expires, CNHs must renew the notification within 1 month of the expiry date.
With the rise in demand for safe cosmetic products and other significant regional trends (such as halal products and vegan products), the Malaysian cosmetics market is expected to see a rapid growth in the coming years. The scenario may create a pool of opportunities for manufacturers to tap into, for which comprehending the NPRA regulations is the only way out for successful compliance. How informed are you? Gain access to on-time regional Regulatory insights.









