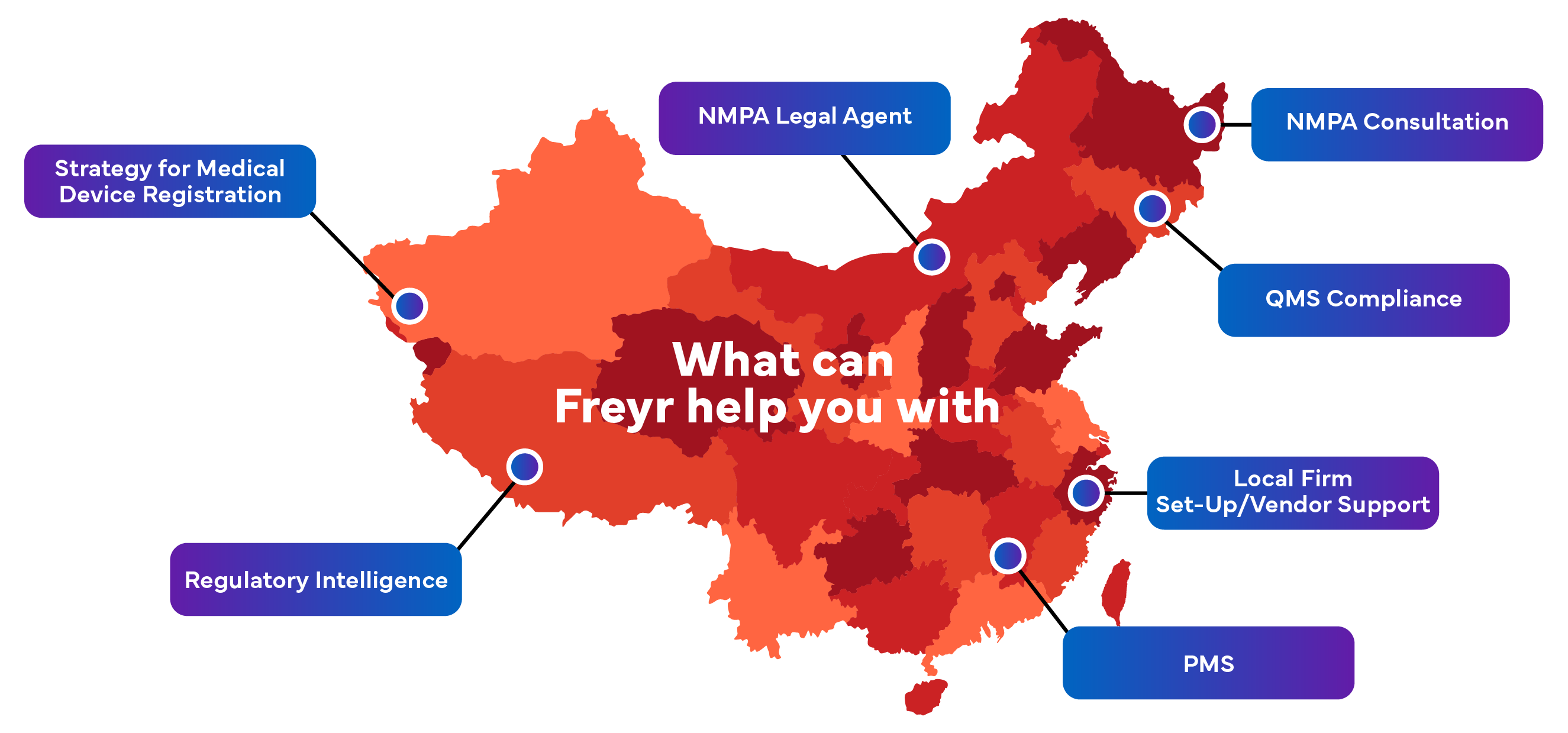
WHY FREYR?
Get seamless market access with Freyr as your China NMPA agent. Freyr represents many global Medical Device company as China Agent for NMPA registration.
Meet our Regulatory Experts
Book a meeting
with our regulatory experts today to achieve swift compliance with all China Medical Device regulations.
Frequently Asked Questions (FAQs)
- Regulatory Authority: NMPA (National Medical Products Administration) (formerly CFDA)
- Regulation: State Council Order No.739
- Authorized Representative: NMPA Legal Agent Required
- QMS Requirement: YY/T0287-2017, ISO 13485:2016
- Assessment of Technical Data: Centre for Medical Device Evaluation (CMDE)
- Labelling Requirements: Decree No.6 of CFDA
- Submission Format: eRPS
- Language: English & Chinese
The device classification is defined in the National Medical Products Administration’s (NMPA’s) Medical Device Classification Catalogue (Announcement No. 104/2017), * or/and the rules in Order No. 15 for medical devices. The devices are classified into three (03) classes based on the risk criteria. Class I devices are low-risk devices and Class III devices are high-risk devices.
| Device Class | Risk |
| I | Low-risk |
| II | Medium-risk |
| III | High-risk |
For Medical Device Registration in China, for Class I Medical Devices, record-filing with NMPA is required, and for Class II and Class III, registration certificates from NMPA have to be obtained. Class I devices undergo administrative review, whereas Class II and Class III devices undergo a thorough review process. The data and testing requirements vary based on the availability of predicates. Hence, Class II and Class III device manufacturers should also identify predicates to determine the clinical data requirements for their devices. The NMPA issues Record Filing and Registration Certificates for Class I and Class II/III devices, respectively.
In Summary, for NMPA Registration-
Class I- Registration Dossier and Administrative Review
Class II- Full Registration Dossier and Full Application Review (including technical)
Class III- Full Registration Dossier and Full Application Review (including technical)
The Chinese medical device regulation defines the following registration process for medical device approval in China-