
On May 27, 2016, the United States Food and Drug Administration (US FDA) published the final rule for nutrition and supplement facts label in the Federal Register. The final rule ensures that the label reflects new scientific information including the link between diet and chronic diseases like obesity and heart disease. The new rule intends to ensure that the product label is in line with current food habits and practices which are not clearly established in the old label rule. Another significant update of the rule is that the FDA has declared compliance deadlines basing on the annual food sales of the organization.
Compliance Deadlines
Nutraceutical manufacturers aiming the USA market entry must adhere to the new label rule by the specified deadline failing to do which, they may face product recalls. The deadlines are:
- January 1, 2020 for manufacturers with $10 million or more in annual sales
- January 1, 2021 for manufacturers with less than $10 million in annual sales
Are you aiming for USA market entry? Evaluate your Regulatory procedures.
Major Changes of Nutrition Facts Label
To comply with the new rule, it is required that stakeholders decode the revised rule and abide by it accordingly. The revised rule includes but is not limited to the changes listed below.
- Eliminate the declarations of ‘Calories from fat’
- Ensure declaration of grams (g) of ‘added sugars’ in a serving of the product
- Establish Daily Reference Value (DRV) for added sugars and declare the percent Daily Value (DV) for the same
- Change ‘Sugars’ to ‘Total Sugars’ and declare “Including ‘X’ grams Added Sugars” directly below ‘Total Sugars’
- Update the list of vitamins and minerals of public health significance
- Update reference values used in the declaration of present DVs of nutrients on Nutrition Facts labels
- Revise format of Nutrition Facts labels to increase the prominence of the declaration of ‘Calories’
- Remove requirement of footnote table listing the reference values for certain nutrients for 2000 and 2500 calorie diets
- Maintain records to support the declaration of certain nutrients under specified circumstances
- Amend the definition of single-serving container
- Require dual-column labeling for certain packages
- Amend reference amounts customarily consumed that are used by manufacturers to determine their label serving size
To gain more insights as to how the new label shall appear, the FDA has provided side by side comparison of new and old labels. Below provided is the adaptation of the same.
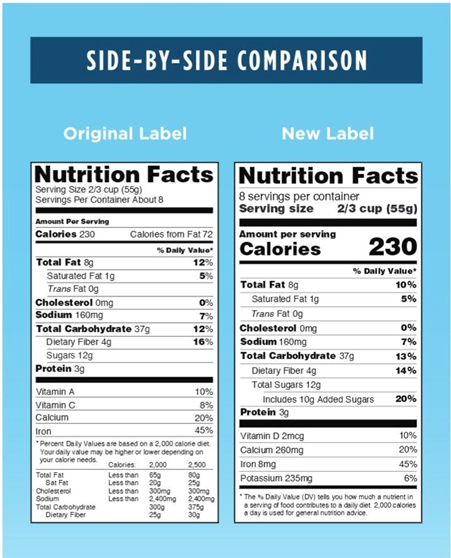
As the deadline for final nutraceutical and supplement facts label rule is approaching closer, manufacturers and other stakeholders should plan for compliance with the assistance of dedicated labeling expert. Ensure your product label is reviewed within time. Be informed. Be Compliant.









