
In today’s highly regulated pharmaceutical landscape, maintaining Good Practice (GxP) compliance is not just a legal necessity, it's a commitment to patient safety and product quality. As Regulatory authorities across the globe, including the FDA and EMA, tighten their focus on manufacturing controls and product consistency, Continuous Process Verification (CPV) has emerged as a transformative methodology for ensuring ongoing GxP compliance throughout the product lifecycle.
Understanding Continuous Process Verification
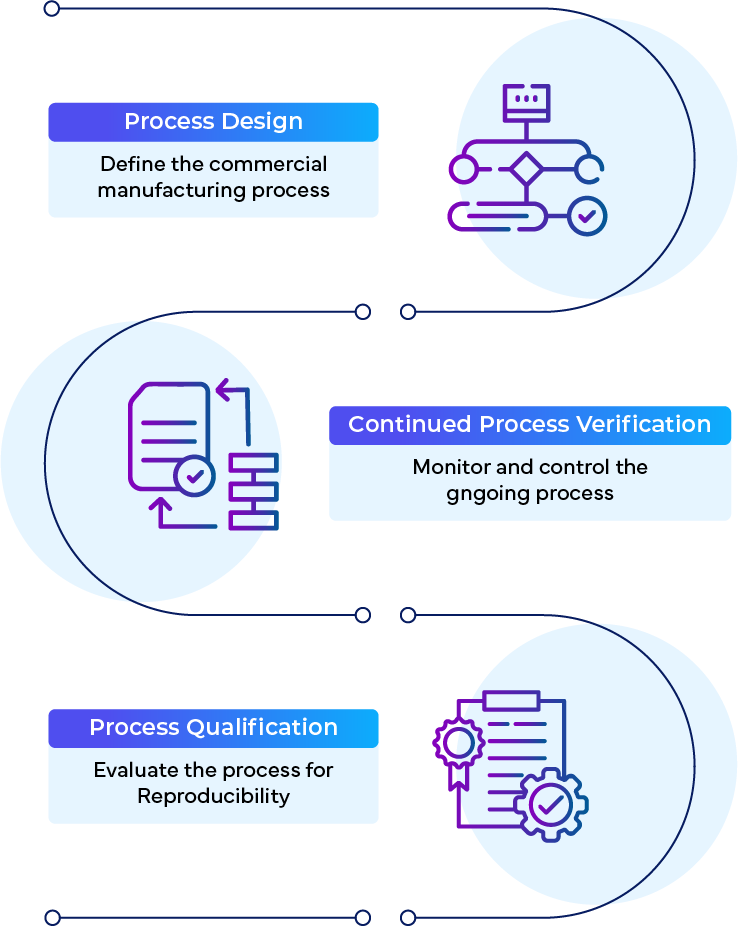
CPV is one (1) of the three (3) stages in the FDA’s Process Validation guideline:
- Process Design
- Process Qualification
- Continued Process Verification
Unlike traditional validation approaches, which often rely heavily on limited data from pre-market batches, CPV emphasizes real-time data collection and trend analysis during routine production. This shift from reactive to proactive quality management allows manufacturers to detect process deviations, mitigate risks early, and maintain a validated state throughout the product's commercial life.
Why CPV Is a Game-Changer
- Real-Time Quality Assurance
By collecting and analyzing data during every production run, CPV facilitates the immediate detection of anomalies. This real-time insight enables quicker decisions, reducing the risk of releasing substandard products and preventing costly recalls. - Enhanced Regulatory Compliance
Regulatory authorities are increasingly expecting pharmaceutical companies to implement lifecycle approaches to validation. CPV supports this expectation by demonstrating a continual state of control, aligning with ICH Q8, Q9, and Q10 guidelines, and ensuring alignment with global compliance requirements. - Cost Optimization
Though CPV implementation requires an upfront investment in analytical technologies and training, it ultimately reduces long-term costs. It minimizes the need for frequent re-validation, decreases batch rejections, and improves manufacturing efficiency. - Data-Driven Decision Making
CPV relies on advanced statistical tools and automation to process vast amounts of data. This structured approach to data management aids in strategic planning, continuous improvement, and informed risk management—cornerstones of modern quality systems.
Implementation Best Practices
1. Define Critical Process Parameters (CPPs)
Start by identifying and establishing CPPs and Critical Quality Attributes (CQAs) based on historical and experimental data. These metrics serve as the foundation for real-time monitoring.
2. Leverage Digital Tools and Automation
Integrate CPV software with your Manufacturing Execution Systems (MES) and Laboratory Information Management Systems (LIMS). Automation enhances data accuracy, frequency, and trend visualization.
3. Cross-Functional Collaboration
Successful CPV implementation requires coordination between Quality Assurance, Manufacturing, and IT teams. Establishing a unified CPV strategy ensures consistency and shared accountability.
4. Develop Robust Data Analysis Models
Use statistical process control (SPC), multivariate analysis, and machine learning algorithms to analyze trends, predict deviations, and support continuous improvement.
5. Regulatory Alignment
Document every aspect of your CPV process and ensure your system is audit ready. Align your documentation and practices with global Regulatory expectations.
CPV in Action: A Case Example
Consider a pharmaceutical company manufacturing sterile injectables. Prior to CPV implementation, their validation efforts focused primarily on three (3) pre-market batches. Post-market variations often went unnoticed until product complaints arose.
By transitioning to CPV, the company deployed real-time temperature and pressure sensors across its production line. Data was continuously analyzed using SPC charts. As a result, they identified a minor but recurring drift in fill volume, early enough to take corrective action before the deviation impacted product quality. This shift not only ensured GxP compliance but also significantly reduced downtime and product rework.
Conclusion
Continuous Process Verification is more than a Regulatory checkbox. It’s a strategic approach to ensuring consistent product quality, operational efficiency, and compliance with evolving GxP standards. With the right tools, mindset, and expert guidance, pharmaceutical companies can transform their validation programs and drive sustained compliance.
Let Freyr Guide Your CPV Journey
At Freyr, we specialize in end-to-end Regulatory and compliance services for the pharmaceutical industry. Whether you’re just beginning your CPV implementation or looking to enhance your current system, our experts can help you design, deploy, and validate robust CPV frameworks tailored to your needs.
Ready to elevate your GxP compliance? Contact Freyr today to learn how we can help future-proof your pharmaceutical operations.









