
In the pharmaceutical industry, patient safety hinges on one critical factor, consistency in product quality. Every tablet, vial, or injection that reaches a patient must perform precisely as intended. This consistency is the result of rigorous process validation (PV) governed through a robust Quality Assurance (QA) framework.
Process validation is a quality philosophy that ensures manufacturing processes are well-understood, controlled, and capable of consistently delivering products meeting their predefined specifications. Through the QA lens, process validation transforms from a compliance exercise into a proactive safeguard for both product integrity and patient health.
What is Process Validation?
According to FDA and ICH Q8–Q10 guidelines, process validation is “the collection and evaluation of data, from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering quality products.”
The validation lifecycle typically includes three (3) critical stages:
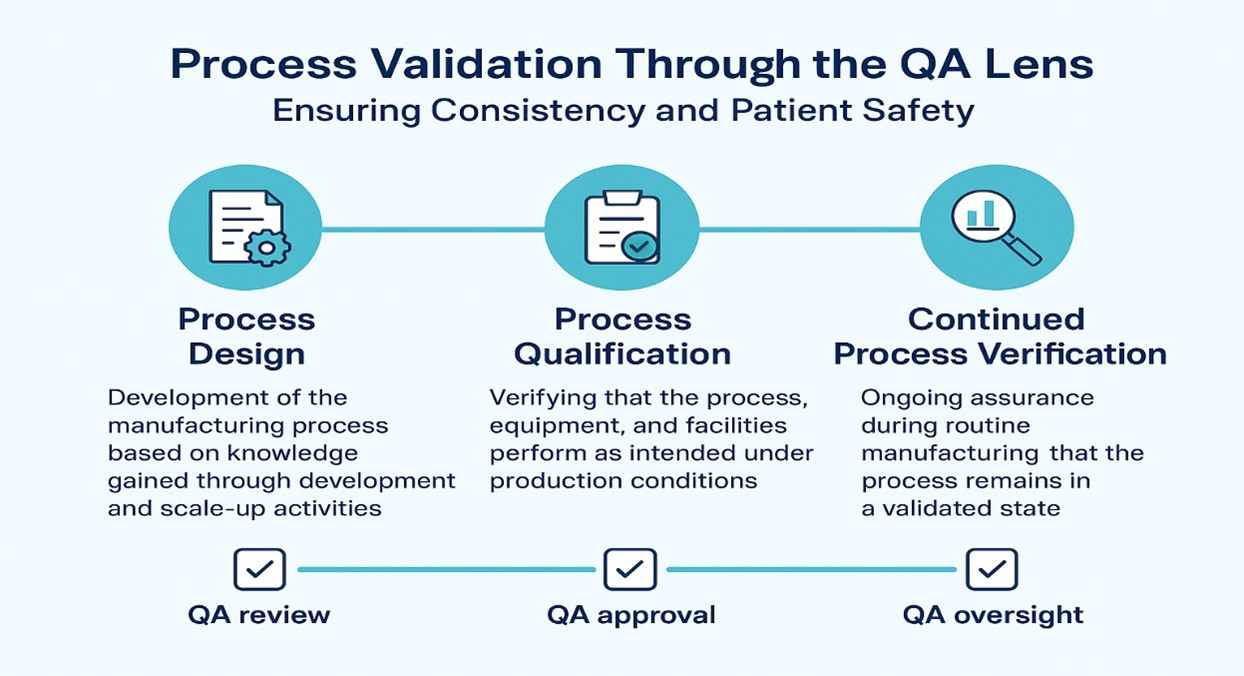
- Stage 1 – Process Design:
Development of the manufacturing process based on knowledge gained through development and scale-up activities.
QA’s role: Ensuring risk assessments, design controls, and quality-by-design (QbD) principles are applied. - Stage 2 – Process Qualification:
Verifying that the process, equipment, and facilities perform as intended under production conditions.
QA’s role: Approving qualification protocols, reviewing deviations, and ensuring data integrity in execution. - Stage 3 – Continued Process Verification (CPV):
Ongoing assurance during routine manufacturing that the process remains in a validated state.
QA’s role: Implementing trend analysis, quality metrics, and CAPA systems to maintain control.
The QA Perspective: Beyond Documentation
While process validation involves technical and statistical evaluations, the QA function ensures it is executed with scientific rigor and Regulatory compliance. QA’s responsibility spans every phase; from planning and protocol approval to change management and ongoing verification.
- Risk-Based Approach
QA plays a pivotal role in adopting a risk-based approach as defined by ICH Q9. Critical Process Parameters (CPPs) and Critical Quality Attributes (CQAs) are identified, assessed, and monitored to minimize risks. QA ensures that validation protocols are statistically sound and risk mitigation plans are embedded into the design.
- Data Integrity and Traceability
In an era of digital transformation, data integrity remains a core focus for QA. The ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) guide every data entry and review. QA ensures that validation data—from equipment qualification to batch records—are traceable, verified, and compliant with 21 CFR Part 11 requirements.
- Continuous Improvement through CPV
Post-validation, QA monitors process performance using statistical process control (SPC) and trending tools. Deviations or out-of-trend results trigger Corrective and Preventive Actions (CAPA) to continuously improve process capability. This closed-loop system strengthens long-term compliance and reduces product variability.
Regulatory Expectations and Global Standards
Regulators worldwide, including the US FDA, EMA, MHRA, and WHO, expect pharmaceutical companies to demonstrate process validation through a lifecycle approach. QA ensures alignment with these evolving guidelines, particularly:
- FDA Guidance for Industry – Process Validation: General Principles and Practices
- ICH Q8 (Pharmaceutical Development), Q9 (Quality Risk Management), and Q10 (Pharmaceutical Quality System)
- EU Annex 15 – Qualification and Validation
By maintaining harmonized documentation and traceability, QA ensures inspection readiness and global Regulatory compliance, irrespective of regional variations.
Process Validation Challenges and QA Solutions
Challenge | QA-Driven Solution |
|---|---|
Inconsistent data across batches | Implement electronic batch records (EBR) and QA data review checkpoints |
Lack of statistical justification in sampling | QA mandates protocol alignment with statistical confidence levels (e.g., 95% confidence intervals) |
Inadequate documentation | QA enforces document control systems and SOP-driven traceability |
Deviations during validation | QA leads deviation handling, root cause analysis, and CAPA implementation |
Lifecycle management | QA ensures CPV programs and revalidation triggers are clearly defined |
Digital Transformation in QA for Process Validation
Modern QA teams are leveraging automation and digital validation platforms to enhance efficiency and accuracy. Tools like Validation Lifecycle Management Systems (VLMS) allow real-time collaboration between QA, manufacturing, and validation teams.
Freyr’s expertise in Computer System Validation (CSV) and Computer Software Assurance (CSA) helps clients adopt compliant, scalable, and inspection-ready digital validation strategies. This ensures not just compliance but also operational excellence and faster product release timelines.
Building Trust Through Quality
Process validation is an ongoing commitment to quality, safety, and patient trust. Through QA oversight, pharmaceutical companies can ensure their processes are not only validated but continuously improved to meet the highest global standards.
By embedding QA into every phase of process validation, organizations strengthen their Quality Management System (QMS), reduce Regulatory risk, and deliver on the ultimate promise—safe and effective products for patients worldwide.
At Freyr, our team of QA and validation experts’ partner with global pharmaceutical companies to establish robust, compliant, and inspection-ready validation frameworks. From QMS remediation and process validation strategy development to digital QA transformation, Freyr ensures seamless Regulatory alignment and operational excellence.
Explore how Freyr can help you achieve validation excellence.









