
In the highly regulated pharmaceutical industry, harmonizing Regulatory compliance across multiple jurisdictions is a pressing challenge. With diverse Regulatory frameworks, varying quality standards, and evolving health authority requirements worldwide, pharmaceutical companies must navigate complexity to ensure seamless Regulatory alignment. Achieving robust global Regulatory compliance is critical for timely market access, maintaining product safety, and ensuring consistent manufacturing and labeling standards.
Challenges in Multi-Jurisdictional Regulatory Harmonization
Pharmaceutical companies face significant obstacles harmonizing compliance internationally. Regulatory authorities, such as the US FDA, EMA, and MHLW, among others, have differing expectations for documentation, inspection, submission timelines, and risk management. These discrepancies lead to redundant efforts, increased operational complexity, and delayed product launches. Additionally, regional differences in cultural, ethical, and technology infrastructures further complicate compliance standardization. Issues surrounding data security and privacy regulations hinder the smooth exchange of data across borders, which is crucial for global Regulatory submissions and audits. Navigating these multilayered challenges requires strategic planning, agile processes, and expert knowledge of global pharmaceutical regulations and standards.
Strategies for Effective Compliance Harmonization
Freyr Solutions’ end-to-end Regulatory compliance services exemplify best practices in addressing cross-jurisdictional harmonization challenges. Their centralized, scalable frameworks align with critical regulations such as 21 CFR Part 11, ICH-GCP, GAMP 5, PIC/S, ISO 27001, and HIPAA, ensuring compliance consistency on a global scale. Key strategies include:
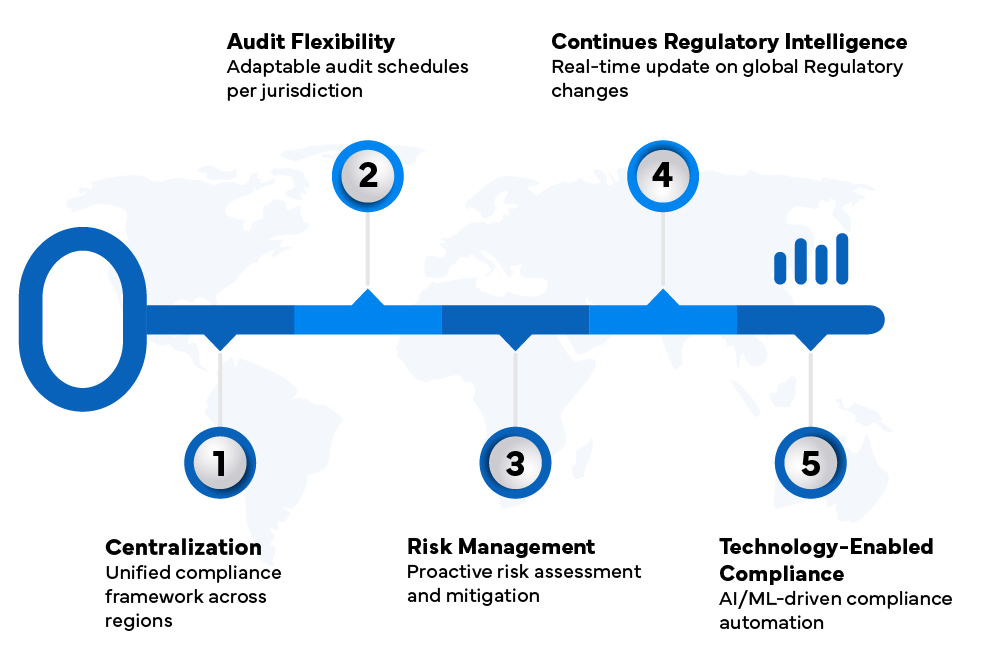
- Centralized Regulatory Compliance Frameworks: Integrating quality management documents, SOPs, training records, and audit findings into a single platform eliminates duplicative work and promotes process uniformity across multiple sites and jurisdictions.
- Flexible Audit Services: Offering remote, nearshore, and on-premises audits tailored to Regulatory expectations optimizes resource use while ensuring audit readiness and compliance.
- Risk-Based CAPA Management: Systematic corrective and preventive actions supported by root cause analysis (RCA) enable swift resolution of compliance deviations, mitigating Regulatory risks internationally.
- Continuous Regulatory Intelligence: Regular monitoring of Regulatory updates and maintaining agency relations allow proactive adjustments to changing compliance requirements, keeping pharmaceutical operations ahead of Regulatory shifts.
- Advanced Compliance Technology: Utilizing validated electronic systems for documentation, training management, and audit readiness enhances efficiency and data integrity, crucial for multi-jurisdictional Regulatory compliance.
Key Strategies for Harmonizing Regulatory Compliance Across Multiple Jurisdictions in Pharmaceuticals
AI/ML-driven compliance automation
Organizations that successfully harmonize Regulatory compliance across countries experience a multitude of benefits. These include accelerated time-to-market through elimination of redundant submissions and audits, improved product quality and patient safety across global markets, and enhanced operational efficiency via standardized processes. Harmonization fosters better collaboration among global teams, suppliers, and authorities by establishing consistent protocols and expectations. Real-time data-driven compliance monitoring through management dashboards supports continuous quality improvement and effective Regulatory risk mitigation.
Conclusion
In a complex global pharmaceutical landscape, harmonizing Regulatory compliance across jurisdictions is essential for sustaining competitive advantage and Regulatory success. Freyr Solutions’ end-to-end Regulatory compliance services provide the industry-leading expertise, scalable frameworks, and technology innovations required to overcome harmonization challenges. By partnering with Freyr, pharmaceutical companies can ensure consistent compliance with global Regulatory standards, streamline audit readiness, and accelerate access to critical medicines for patients worldwide.
Contact Freyr Solutions today to discover how their tailored Regulatory compliance consulting can help your organization achieve seamless multi-jurisdictional compliance and operational excellence in the life sciences industry.









