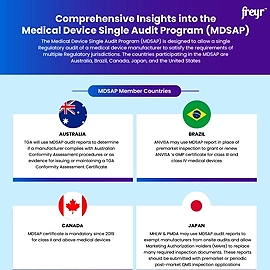
What exactly is the FDA’s new rule for QMSR?
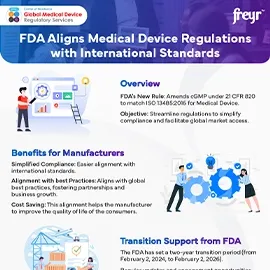
The FDA's new Quality Management System Regulation (QMSR) updates Quality System (QS) Regulation, aligning it with ISO 13485:2016, the global medical device quality management standard. This change aims to harmonize Regulations, ensuring timely access to safe, high-quality medical devices.
The QMSR incorporates ISO 13485:2016 requirements while maintaining consistency with other FDA Regulations. Updates to part 4 (21 CFR part 4) clarify QMS expectations for combination products, without altering cGMP requirements.
The enforcement begins on February 2, 2026, giving time to manufacturers adapt to the QMSR transition.
Focus on the transition period!
With enforcement beginning in 2026, delaying the transition could lead to significant penalties. The two-year window provided to manufacturers is an opportunity to align their Quality Management Systems (QMS) with the new QMSR requirements.
The enforcement begins on February 2, 2026, giving time to manufacturers adapt to the QMSR transition.
Utilize the transition period effectively in 2 simple steps:
Step 1:
Identify gaps, train teams, and begin updates
Step 2:
Audit, finalize changes, and ace compliance!
Our expert team ensures a seamless transition with a tailored 4-step strategy:
- Comprehensive Gap Analysis to identify critical updates
- End-to-End Risk Management aligned with the device lifecycle
- Strengthened Design Controls & Post-Market Surveillance systems
- Staff Training on QMSR requirements & ISO 13485:2016 standard requirements/implementation
Stay ahead of the compliance curve
Frequently Asked Questions
No, ISO 13485 certification aids compliance but does not exempt manufacturers from FDA inspections. Additional steps must be taken to meet QMSR-specific requirements.
The duration depends on your organization’s current QMS maturity and resource availability. A gap analysis is a critical first step to estimate the timeline and plan accordingly.
Transitioning to QMSR compliance ensures your QMS meets FDA requirements and international standards, which helps in reducing Regulatory risks, improving product quality, and improving patient safety.
Immediate action is recommended to ensure a smooth transition before Regulatory deadlines. Starting early provides the necessary time for gap analysis, training, and execution.
Failing to transition to QMSR compliance can have serious business consequences, including:
- Non-compliance during FDA inspections
- Delays in product approvals
- Increased Regulatory inquiry
- Possible fines or recalls
Transitioning proactively safeguards your business against these risks and ensures uninterrupted operations and compliance.
Why Partner with Freyr?
Streamlined Regulatory Compliance
Our QMSR experts guide your organization through a hassle-free transition to the FDA's QMSR, ensuring precision and efficiency
Tailored Compliance Solutions for Your Business
Receive customized strategies that align with your device type and lifecycle, enabling a seamless and successful QMSR transition
Risk Mitigation
We ensure your QMS documentation meets FDA requirements, minimizing compliance risks