
Packaging artworks is more than just designing, it's a compliance mandate that is required for submission to the health authority and even for commercialization of packaging. Within the European Union (EU), pharmaceutical products must adhere to centralized artwork requirements established under Directive 2001/83/EC. However, many EU member states also enforce additional local requirements based on language, national laws, and patient accessibility for their primary and secondary packaging
Understanding these nuances is critical for pharmaceutical companies aiming to ensure regulatory compliance, avoid costly delays, and streamline market entry.
EU-Wide Artwork Requirements: The Foundation
Under Directive 2001/83/EC Article 54, all human medicinal products must display the following information on outer and inner packaging:
- Name of the medicinal product (including strength and pharmaceutical form)
- Active ingredients and quantity
- Route of administration
- Expiry date
- Storage instructions (if applicable)
- Special warnings (if necessary)
- Batch number
- Manufacturer details
- Braille for the name of the medicinal product
These are mandatory across all EU countries, ensuring a consistent baseline for patient safety and information clarity.
Country-Specific Artwork Requirements
Despite harmonized EU regulations, individual countries enforce specific artwork and labeling conditions. Here’s a snapshot of key country-specific requirements:
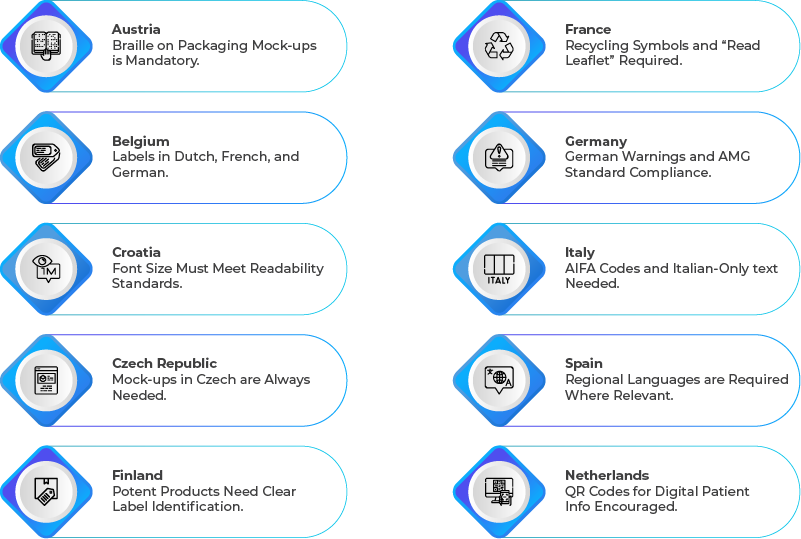
Why Compliance Varies
Each EU country adopts common guidelines to reflect:
- Linguistic diversity (e.g., Belgium, Spain)
- National laws and health authority expectations
- Accessibility considerations like Braille or color contrast
- Patient safety through localized warnings or digital enhancements
How Freyr Solutions Supports EU Artwork Compliance
At Freyr Solutions, we offer specialized Regulatory Artwork Services for the European market, including:
- Multi-language package label development for outer and inner packaging
- Country-specific artworks for submission and commercial purposes
- Braille and Blue Box integration
- Regulatory proofreading and quality checks
- Artwork lifecycle management across EU products
Whether launching a product in one EU country or across the region, Freyr ensures your packaging is audit-ready, accurate, and compliant with both EU and local standards. In addition, adaptive design from one country to another is very seamless.









