
The pharmaceutical industry is steadily embracing digital transformation across functions—from R&D to Regulatory Affairs and Artwork Management Systems (AMS) have become a backbone for managing packaging artworks across global supply chains. They promise transparency, version control, structured workflows, and audit readiness. Yet, even with these sophisticated systems in place, manual decision-making by stakeholders continues to delay artwork changes—a gap that carries significant compliance and supply chain risks.
Let’s explore why technology alone cannot replace human judgment and how the behaviour of people handling these tools continues to shape the efficiency, accuracy, and compliance of artwork operations.
Typical gaps that keep the Artwork Management System (AMS) from delivering full value
From implementations and industry literature, the recurring friction points are:
- Weak trigger governance. No enforced SLA or owner to kick off change requests when regulatory or marketing changes occur. Systems may wait for a manual initiation.
- Poor alerting and escalation. AMS configurations often lack proactive reminders, predictive bottleneck warnings or real-time KPIs that force early action.
- Siloed systems. LMS, RIM, AMS and ERP don’t always integrate, so regulatory updates don’t automatically flow into artwork change requests.
- Manual checkpoints that matter. Strategic decisions — such as market prioritization, branding trade-offs, and local RA interpretations — still require human judgment beyond automated rules.
- Limited adoption & process discipline. Users sometimes bypass the system (email threads, spreadsheets), undermining the AMS audit trail and workflow logic.
A composite case: how a delay ripples downstream
Consider a composite (anonymized) scenario common in audits:
A local RA team receives an urgent label guidance update affecting a single market. They review it but do not immediately create a change request in the AMS — instead, they email marketing and note the change in a shared drive. Two weeks pass. The artwork studio receives a late AMS request with compressed timelines. Proofreading overlaps with printing slot bookings; the print vendor has limited capacity and cannot accommodate late changes without premium charges and lead-time extensions. The product misses its planned launch window in that market; parallel packaging that has already been manufactured must be reworked or quarantined. The result: emergency expedited print runs, extra OPEX, market unavailability for several weeks, and an audit finding for poor change control.
This composite mirrors real patterns described in recall and quality analyses: missing or late labeling updates are a common root cause of downstream release and safety issues.
Where Manual Decision-Making Still Dominates
Even in highly automated environments, several artwork activities still require judgment, interpretation, and coordination of stakeholders that tools cannot fully automate.
| Artwork Stage | Example Manual Decision | Why It’s Still Human-Dependent |
| Change Request Evaluation | Deciding if a regulatory update impacts artwork or labeling | Requires contextual understanding of labeling hierarchy and regional differences |
| Change Impact Assessment | The supply chain may or may not align with packaging availability dates. | Stakeholders must ensure the printing partner, transportation and the manufacturing team are aligned to their individual dates before committing |
| Task Assignment & Prioritization | Allocating tasks across markets or teams | System rules can’t always account for workload, expertise, or market urgency |
| Proofing & Approval | Determining if color, spacing, or alignment meet brand/regulatory standards | Visual evaluation and judgment remain subjective |
| Deviation Management | Handling non-standard or urgent artwork requests | Needs cross-functional negotiation beyond workflow logic |
| Final Sign-off | Approving print files or PDFs | Regulatory and QA representatives must use discretion before release |
Table 1: Manual Decision-Making vs. Artwork Management Systems
Why manual intervention will remain — and how to make it work
Process automation can and should reduce repetitive work; however, decision points in the pharmaceutical industry are often intentionally human, including regulatory interpretation, branding trade-offs, and QA risk assessments. The goal is not to eliminate people, but to coordinate them so that decisions are made in a timely manner.
Practical measures to close the gap:
- Define clear SLAs & RACI for triggers. Who must initiate a change request when RA guidance changes? Set max time-to-initiate and monitor adherence.
- Add intelligent alerts and dashboards. Configure the AMS to send pre-deadline reminders, show impending bottlenecks, and highlight critical path tasks. Combine with periodic management dashboards. manageartworks.com
- Integrate systems end-to-end. Link RIM/LMS → AMS → ERP so that regulatory updates generate preliminary change requests or, at the very least, flag impacted SKUs. Asia Regulatory Conference | IFPMA+1
- Operationalize human checkpoints. Embed mandatory, timestamped decision steps in the workflow (e.g., RA ignition approval) so the AMS records both action and accountability.
- Train & incentivize adherence. Make timely initiation part of performance metrics; celebrate Right-First-Time (RFT) wins to change behavior.
- Run post-mortems and measure RFT. Track where delays occur (input, design, proofreading, approvals) and apply continuous improvement.
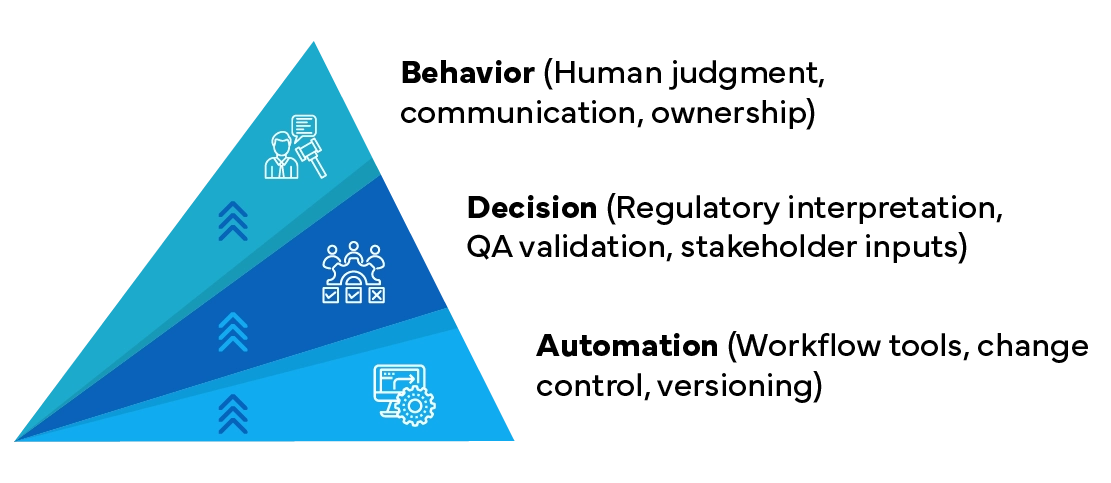
“Human Influence on Artwork Operations under Process Automation Tool”
The pyramid represents the hierarchy of control and influence within artwork operations—starting from technology at the base and progressing to human behavior at the top. While automation provides the foundation for structured workflows, it’s the decisions made by individuals at the upper layers that ultimately determine the success or failure of the process.
Conclusion
Even with cutting-edge Artwork Management Systems, pharma artwork operations cannot be fully automated—because they operate at the intersection of compliance, creativity, and collaboration.
Human judgment remains vital for interpreting regulations, ensuring patient-centric design, and addressing market-specific nuances. However, unstructured decision-making and inconsistent behavior can offset the very efficiency automation aims to deliver.
Ultimately, the true power of an AMS lies not only in its features but also in the discipline, awareness, and accountability of those who utilize it.









