
The artwork lifecycle plays a pivotal role in ensuring that the right product information reaches the right market — accurately, safely, and on time.
However, for many global pharmaceutical companies, artwork management still remains fragmented across geographies and business units, resulting in delays, inconsistencies, compliance risks, and higher operational costs.
To overcome these challenges, leading organizations are embracing three interconnected principles: Standardization, Centralization, and Harmonization of artwork lifecycle processes.
Let’s explore how these three (3) pillars transform artwork operations into a scalable, compliant, and globally consistent function. Together, these initiatives drive measurable outcomes that help in achieving zero non-compliance, zero product recalls, 100% right first-time quality and delivery.
1. Standardization: Building Consistency and Control
Objective:
Creation of uniform procedures, templates, and workflows across global operations, multiple markets and product portfolios
Why It Matters:
When every market operates with its own processes and templates, discrepancies in artwork quality, labeling interpretation, and versioning control become inevitable. Standardization creates a single baseline for compliance and efficiency.
Key Elements of Standardization:
- Global SOPs and Work Instructions for artwork creation and approval.
- Standardized Templates (carton, label, Leaflet layouts or grids, technical specifications templates with barcodes, and braille placements).
- Establishing standard KPIs
- Unified Naming Conventions, branding color usage and Metadata Structures.
- Defined Checklists for proofing, QA, and version verification.
- Using common tools across all vendors
| Parameter | Without Standardization | With Standardization |
| Template Usage | Varied per market | Single validated global template library |
| Review Cycles | Multiple reworks | Reduced iteration and faster approval |
| Error Rate | High (manual dependency) | Low (checklists and automation) |
| Compliance Risk | Increased due to inconsistency | Controlled and auditable |
Table 1: Impact of Standardization on Artwork Efficiency
2. Centralization: Strengthening Oversight and Efficiency
Objective:
Centralization involves consolidating artwork operations under a centralized governance model for efficiency, visibility and collaboration
Why It Matters:
Centralization eliminates silos, ensures traceability, and enhances collaboration between multiple artwork stakeholders for commercial and submission requirements.
Key Elements of Centralization:
- Create a centralized Artwork Center of Excellence that oversees artwork lifecycle management, design and proofreading
- Maintain a Single Source of Truth for all artworks, technical documents, templates, images, fonts, etc.
- Utilize a Artwork Management System (AMS) for all artwork change controls, approvals and version management
- Consolidate vendor management including external design studios and print vendors
- Improved Governance by enabling metrics tracking such as RFT, approval time across all product and regions
Impact of centralization on Artwork Operations
- Single source of truth
- Faster TAT
- Strong Governance
- Global visibility on artwork projects and performance indicators
- No hindrances to scalability requirements
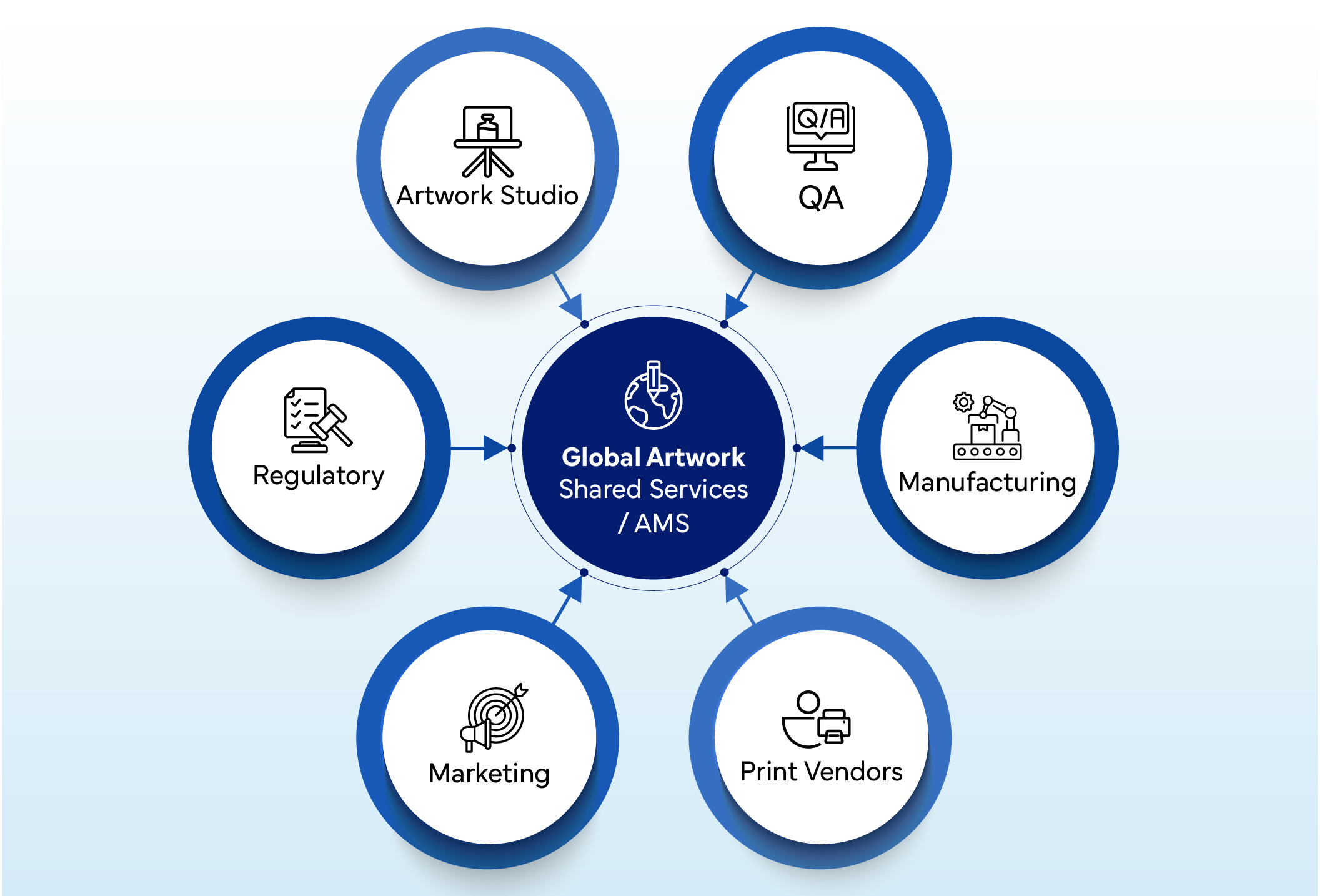
“A centralized artwork hub ensures consistency, speed, and control by connecting all regional functions through a unified platform.”
3. Harmonization: Achieving Global Alignment
Objective:
Aligning of regional practices, process workflows, tools and templates to create a globally coherent artwork management framework — without compromising local regulatory needs, that eliminates duplication
Key Elements of Harmonization:
- Aligning regional artwork processes with global framework to ensure process equivalence
- Harmonize central branding practices to streamline localization
- Harmonizing proofreading and design tools across all sites and vendors for unified quality control
- Harmonize technology platforms such as AMS, DAM for seamless integration with regulatory and commercial functions
Benefits of Harmonization:
- Unified interpretation of global labeling and artwork practices
- Reduction in duplication of market-specific artworks.
- Easier management of shared packs across multiple regions.
- Improved collaboration between stakeholders and better control during regulatory changes or product extensions.
- Simplified compliance management
The Power of Integration: When All Three Work Together
The ultimate goal for pharmaceutical companies is to achieve an integrated Artwork Excellence Framework that combines the strength of all three pillars.
Together, they create a robust artwork management ecosystem capable of supporting rapid product launches, multi-market submissions, and strict regulatory audits.
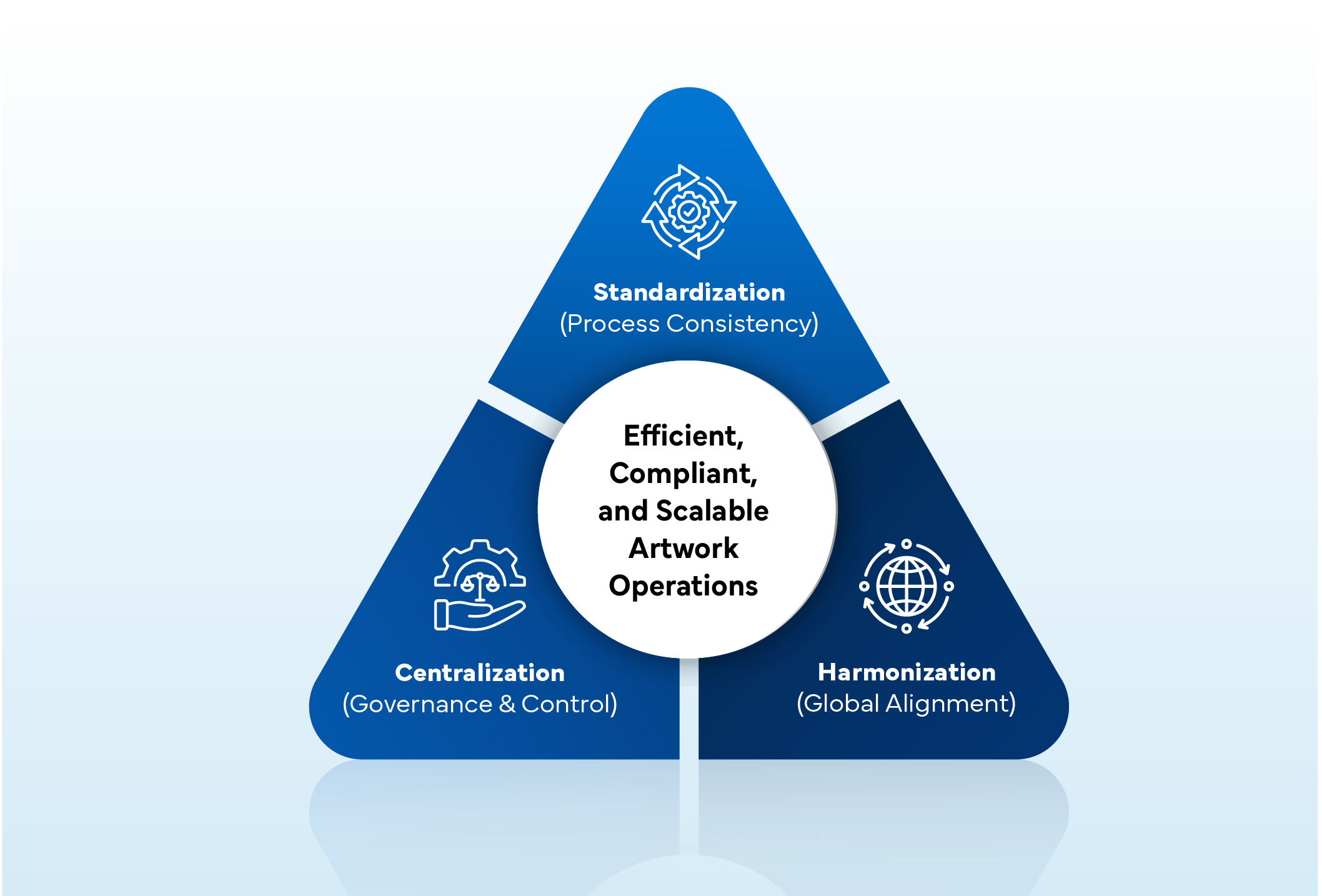
Artwork Excellence Triangle
“When standardization, centralization, and harmonization converge, organizations achieve true artwork excellence — enabling faster, compliant, and consistent packaging delivery.”
Conclusion
In the evolving regulatory landscape, pharma packaging artwork has become more than a compliance formality — it’s a strategic operational function.
To stay competitive and audit-ready, global life sciences organizations must build a foundation rooted in:
- Standardization that establishes consistent processes, templates, KPIs across global operations
- Centralization for consolidating artwork creation, proofreading and lifecycle management activities supported by Artwork Management System (AMS) to enhance visibility, control and visibility
- Harmonization to ensure global coherence, align workflows and achieve local compliance to eliminate redundancies.
When these three pillars align, companies unlock operational agility, regulatory precision, and brand consistency — the hallmarks of an optimized artwork lifecycle.









