
Introduction
Pharmaceutical packaging artworks are critical components in ensuring regulatory compliance, patient safety, and brand consistency. Given the complexity of artwork lifecycle management, traditional methods relying on manual communication, emails, and ad-hoc approvals often result in inefficiencies, compliance risks, and prolonged timelines. This necessitates a structured, technology-driven approach to Artwork Change Workflows enabled in the Artwork PLM system to streamline operations, enhance traceability, and ensure compliance with evolving regulatory standards such as 21 CFR Part 11 and EU Annex 11.
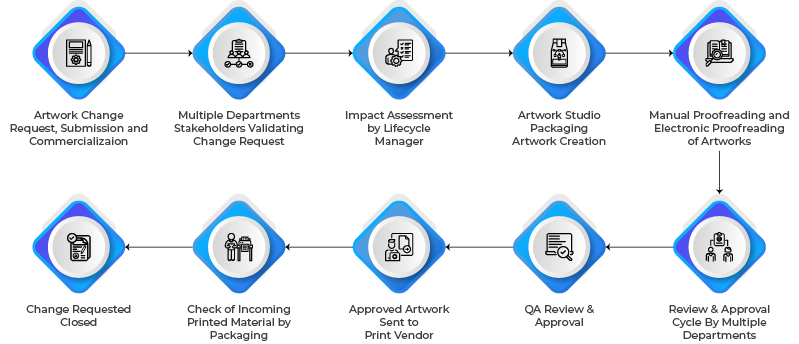
Understanding Artwork Change Workflows
Process Workflow is a structured approach to managing the end-to-end artwork lifecycle through various change workflows designed to manage different artwork requirements, from design inception to final printing, while ensuring compliance with regional and global regulatory requirements. This involves multiple stakeholders, including Regulatory Affairs, Quality Assurance, Marketing, and Packaging teams collaborating efficiently to develop and approve pharmaceutical artwork that adheres to industry guidelines.
Key Components of an Artwork Process workflow tool
1. Regulatory-Driven Artwork Lifecycle Management
- Starting from mock-up design process to submission-ready artwork creation, developed using a specific workflow meant for submission. The approval process might involve individuals from the regulatory affairs team.
- Commercial Workflows can be divided into Launch and Post-marketing artwork change processes. They will require approvals beyond regulatory and will need participation from Supply Chain, Packaging, Quality, and marketing teams.
- Workflows can also be defined for processes such as Packaging Keyline Creation, Brand Guidelines, and even for closing the artwork lifecycle.
2. Automating Artwork Approval and Electronic Proofing
- AI-powered proofreading tools ensure accuracy in typography, barcodes, and even the interpretation of Braille.
- Digital annotation features enable real-time collaboration and feedback from cross-functional teams.
3. Enhanced Traceability and Version Control
- Audit Trails: Secure tracking of all artwork modifications, approvals, and version updates in compliance with FDA, EMA, and MHRA guidelines. Even digital tracking of each workflow step.
- Version Tracking: Ensures that only the latest approved version is used for final printing, reducing the risk of outdated labeling reaching the market.
4. Collaboration Between Stakeholders
- Role-based access control ensures that only authorized personnel approve, modify, or validate artwork files.
- Automated alerts and task assignments eliminate communication bottlenecks and improve workflow efficiency.
Advantages of Implementing an Artwork Process Workflow System
- Regulatory Compliance and Risk Mitigation
- Ensures alignment with regulatory bodies such as the FDA, EMA, TGA, PMDA, and SFDA artwork requirements.
- Reduces the risk of non-compliance penalties, recalls, and legal ramifications.
- Operational Efficiency and Cost Reduction
- Minimizes manual interventions, approval delays, and redundant artwork modifications.
- Accelerates time-to-market by streamlining artwork validation and approval processes.
- Data-Driven Decision-Making
- Customizable reports provide insights into approval timelines, compliance status, and efficiency metrics.
- Facilitates continuous improvement through data-backed process optimization.
Conclusion
As regulatory requirements become increasingly stringent, Artwork Management Systems with customized Workflows are no longer a luxury but a necessity for pharmaceutical companies. Leveraging Freyr Artwork Systems with workflows, organizations can streamline their artwork lifecycle, ensure compliance, and enhance operational efficiency. With features such as traceability, real-time collaboration, version tracking, and proofreading, Freyr’s solution enables companies to meet market demands while maintaining the highest standards of accuracy and regulatory adherence.
To know more about Freyr's artwork process workflow solutions, contact our Artwork Consulting Expert for a one-on-one meeting.
Note: Freyr has all the Services as well as technology-based solutions to improvise your artwork management regulatory requirements.









