
Patient safety is the cornerstone of pharmaceutical operations. Regulatory authorities worldwide expect companies to demonstrate that product quality, safety, and efficacy are maintained throughout the product lifecycle. At the center of this expectation lies audit-driven Quality Assurance (QA), a proactive approach that uses audits as a compliance requirement and as a strategic tool to prevent risks and protect patients.
When effectively implemented, audit-driven QA shifts organizations from reactive issue management to continuous quality improvement, ensuring that patient safety is embedded into every process.
Why Audits Are Critical to Quality Assurance
Audits are systematic, independent examinations designed to verify compliance with GxP, cGMP, and Regulatory requirements. From internal audits to supplier audits and Regulatory inspections, audits provide critical insights into process effectiveness, data integrity, and risk exposure.
Audit-driven QA goes beyond checklist compliance by:
- Identifying gaps before they impact product quality
- Evaluating the effectiveness of quality systems and controls
- Strengthening accountability and quality ownership
- Supporting Regulatory inspection readiness
Rather than viewing audits as isolated events, leading organizations integrate them into their QA strategy to drive patient-centric outcomes.
Connecting Audit Findings to Patient Safety
Every audit observation, whether related to deviations, documentation gaps, training deficiencies, or data integrity, has an impact on patient safety. Audit-driven QA ensures that such findings are assessed through a risk-based lens, prioritizing issues that could affect product quality, safety, or supply continuity.
Key patient safety benefits include:
- Early detection of process failures that could lead to contamination or mix-ups
- Improved control over critical quality attributes and critical process parameters
- Reduced risk of recalls, shortages, and adverse events
- Stronger compliance with pharmacovigilance and post-market requirements
By linking audit outcomes directly to quality risk management, QA teams ensure that patient safety remains the primary focus, not just Regulatory compliance.
From Audit Observations to Effective CAPAs
One of the most critical roles of audit-driven QA is ensuring that Corrective and Preventive Actions (CAPAs) are not only implemented but are effective and sustainable.
A strong audit-driven QA framework:
- Performs robust root cause analysis
- Aligns CAPAs with QMS processes
- Tracks CAPA effectiveness over time
- Prevents recurrence of similar issues
Poorly managed CAPAs are a common trigger for repeat audit findings and Regulatory scrutiny. Audit-driven QA ensures that lessons learned translate into meaningful system improvements that protect patients in the long term.
Integrating Audits into the QMS Ecosystem
Audits are most effective when fully integrated into the Quality Management System (QMS). This integration ensures seamless linkage between audits, deviations, CAPAs, change control, training, and document management.
An integrated approach enables:
- Centralized visibility of compliance risks
- Trend analysis across sites and functions
- Consistent responses to Regulatory expectations
- Improved data integrity and traceability
For organizations undergoing growth, mergers, or Regulatory remediation, QMS establishment and remediation supported by audit-driven QA play a vital role in restoring and sustaining compliance.
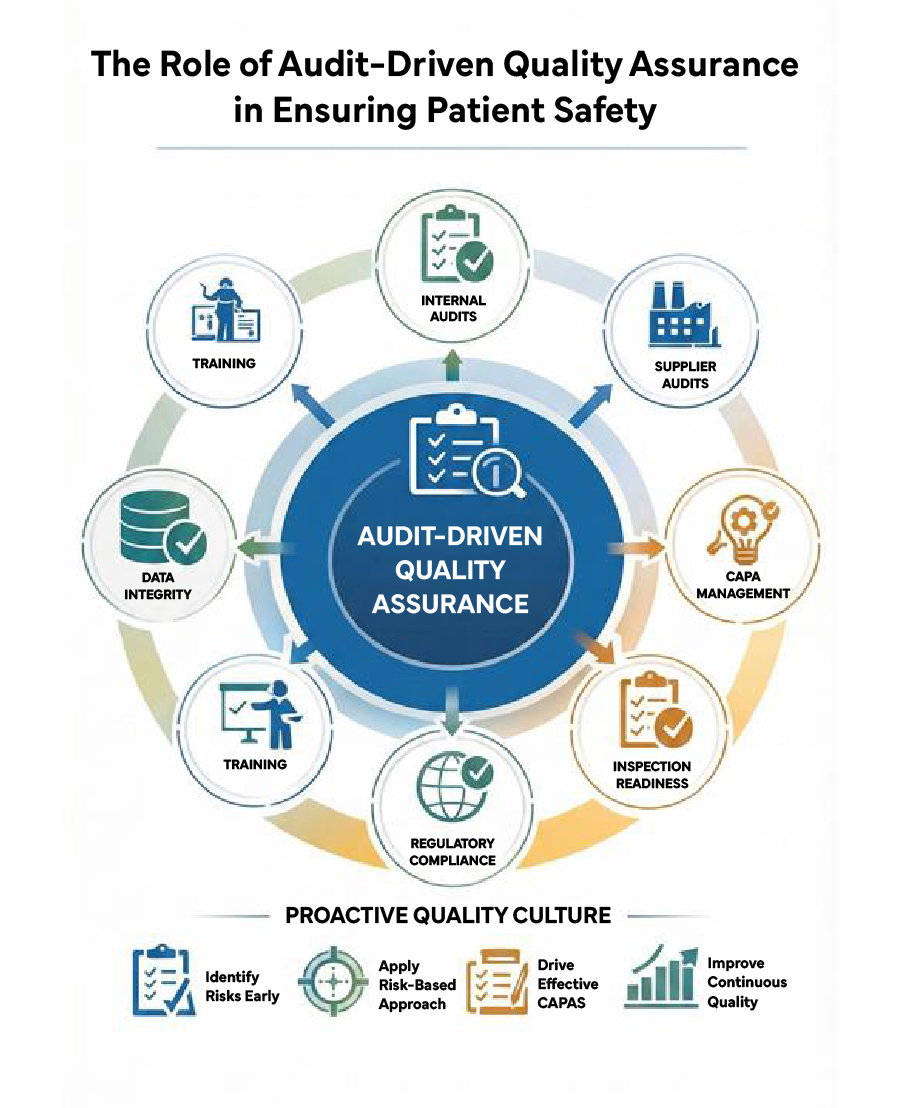
Building a Proactive, Patient-Centric QA Culture
Organizations that excel in audit-driven QA share common best practices:
- Risk-based audit planning aligned with patient impact
- Regular internal and supplier audits
- Strong QA governance and audit readiness programs
- Digital tools and eQMS for tracking findings and trends
- Continuous training and quality awareness initiatives
By embedding audits into everyday quality operations, companies move from compliance-driven behavior to patient-centric quality excellence.
How Freyr Solutions Supports Audit-Driven QA
Freyr Solutions partners with pharmaceutical and life sciences companies to strengthen audit-driven Quality Assurance frameworks through:
- Internal, supplier, and Regulatory audit support
- QMS establishment and remediation services
- CAPA management and root cause analysis
- Inspection readiness and validation services
- SOP writing, review, and quality documentation support
Strengthen your audit-driven Quality Assurance strategy with Freyr Solutions and ensure patient safety through proactive compliance. Connect with our QA and compliance experts today.









