
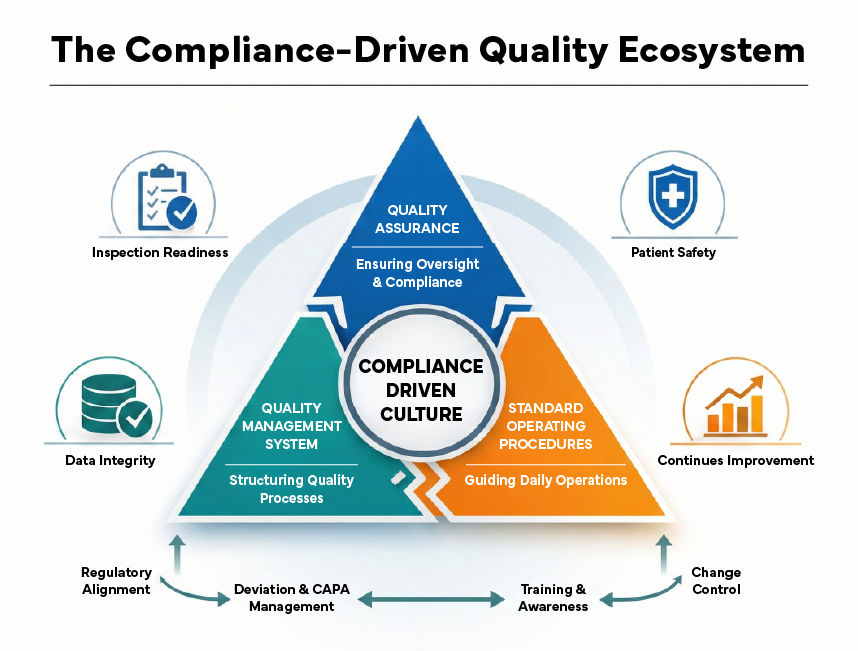
In today’s highly regulated pharmaceutical and life sciences environment, compliance is a continuous, organization-wide commitment. Regulatory authorities expect companies not to demonstrate compliance on paper, but a sustainable quality culture embedded in daily operations. At the heart of this culture lie three interconnected pillars: Quality Assurance (QA), Quality Management Systems (QMS), and Standard Operating Procedures (SOPs).
When these elements function in silos, organizations face audit findings, inspection delays, and operational inefficiencies. When aligned effectively, they create a compliance-driven culture that supports inspection readiness, product quality, and patient safety.
Quality Assurance: The Oversight That Safeguards Compliance
Quality Assurance is the strategic function that ensures pharmaceutical products consistently meet Regulatory, safety, and quality standards. QA provides independent oversight across the product lifecycle, from development and manufacturing to distribution and post-market activities.
A strong QA framework:
- Ensures adherence to GxP, cGMP, and global Regulatory requirements
- Drives quality risk management and deviation handling
- Oversees audits, CAPAs, and change control
- Promotes continuous improvement and inspection readiness
However, QA cannot operate in isolation. Without structured systems and clearly defined procedures, QA oversight becomes reactive rather than proactive.
QMS: The Structural Backbone of Compliance
A Quality Management System (QMS) provides the formal structure that governs how quality activities are planned, executed, monitored, and improved. Whether paper-based or digital (eQMS), QMS integrates critical processes such as document management, training, deviations, CAPA, audits, and risk management.
An effective QMS:
- Standardizes quality processes across sites and regions
- Enables traceability and data integrity
- Supports Regulatory inspections and audits
- Facilitates global compliance and harmonization
For organizations facing Regulatory findings or rapid growth, QMS remediation and establishment become essential to restore compliance and build long-term resilience. Yet even the most robust QMS depends on well-written and consistently followed SOPs.
SOPs: Translating Compliance into Daily Practice
Standard Operating Procedures (SOPs) are where strategy meets execution. SOPs translate Regulatory expectations and QMS requirements into clear, actionable instructions for employees.
Well-designed SOPs:
- Define roles, responsibilities, and workflows
- Reduce variability and human error
- Enable effective training and knowledge transfer
- Demonstrate compliance during inspections
Poorly written, outdated, or inconsistent SOPs are among the most common root causes of audit observations. SOP writing, review, and rationalization are therefore critical to maintaining inspection-ready operations.
Where QA, QMS & SOPs Converge
A compliance-driven culture emerges when QA, QMS, and SOPs function as an integrated ecosystem rather than standalone components.
- QA defines what must be achieved
- QMS defines how it is governed
- SOPs define how it is executed daily
This intersection ensures:
- Regulatory requirements are embedded into processes
- SOPs are aligned with QMS workflows and QA oversight
- Deviations, CAPAs, and changes are managed consistently
- Employees understand not just what to do, but why it matters
Building and Sustaining a Compliance-Driven Culture
Organizations that successfully integrate QA, QMS, and SOPs share common best practices:
- Regular QMS gap assessments and remediation
- Periodic SOP reviews aligned with Regulatory updates
- Strong QA governance and audit readiness programs
- Training programs that reinforce quality ownership
- Digitalization through eQMS and document management systems
Ultimately, compliance becomes a shared responsibility, not just a QA function.
How Freyr Solutions Can Help
Freyr Solutions supports pharmaceutical and life sciences companies in building, remediating, and sustaining compliance-driven quality systems through:
- Quality Assurance and audit support
- QMS establishment, remediation, and eQMS implementation
- SOP writing, review, rationalization, and integration
- Inspection readiness and Regulatory compliance services
Partner with Freyr Solutions to transform QA, QMS, and SOPs into a unified compliance framework that strengthens inspection readiness, operational efficiency, and patient safety. Connect with our compliance experts today.









