Content to Carton - Overview
In life sciences, “Content to Carton” defines the seamless transition of regulatory-approved label content into compliant packaging artwork and ultimately into printed cartons. This process involves:
- Ensuring regulatory labeling and packaging artwork guidelines are met for global compliance.
- Creating artwork content that is both compliant and market-ready for global audiences.
- Producing packaging artwork design aligned with safety standards and health authority (HA) requirements.
- Printing cartons that are consistent, accurate, and aligned with artwork guidelines for packaging.
- Leveraging domain expertise across the artwork lifecycle management process.
With globalization and multi-market expansions, pharmaceutical manufacturers face challenges in visibility and coordination across submission to commercial artwork processes. Freyr’s artwork management consulting bridges these gaps with a right-first-time approach.
Process Steps & Functions Involved in Commercial Request
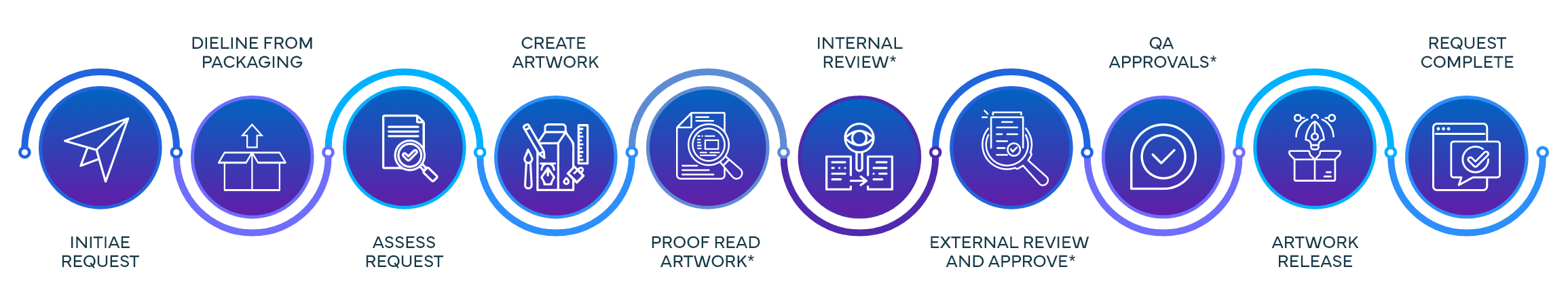
Process Steps & Functions Involved in Submission Request
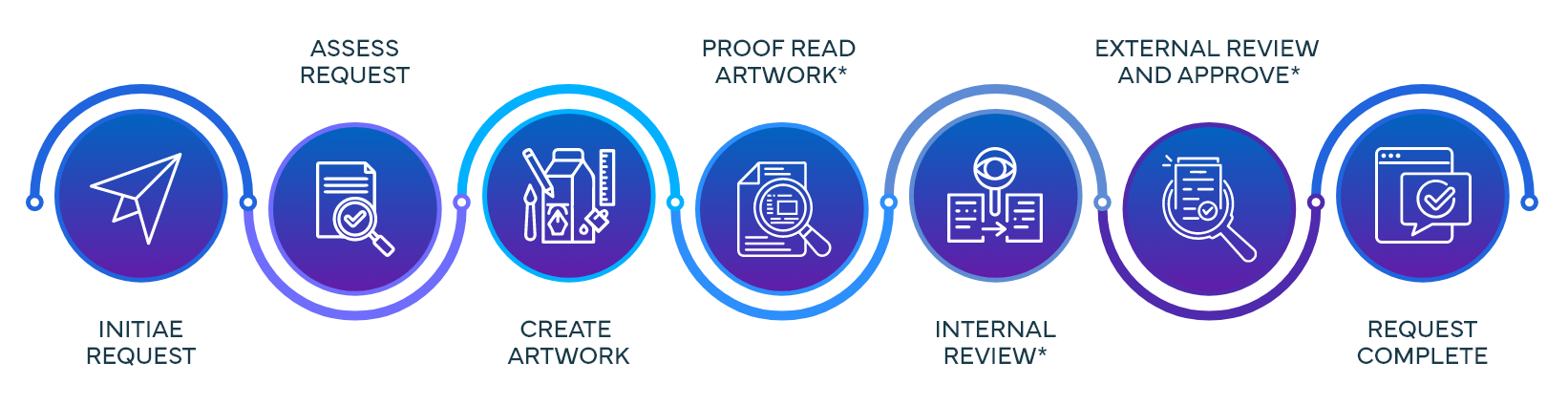
Content to Carton
- Content creation for labeling and artwork (including CCDS to local labeling content).
- Pharmaceutical artwork management for submissions and commercialization.
- Content submission to Health Authorities (HAs) as part of the dossier process.
- Submission of sample artwork and artwork change management based on HA reviews.
- Creation of commercial artworks for local markets with integration of regulatory and technical change specifications.
- Artwork studio services, proofreading, and e-proofing support.
- Multi-level review and approval (Regulatory, Marketing, Packaging, Quality).
- Valued partnerships for artwork printing and packaging artwork design.
- 24/7 operational support for regulatory artwork services.

- Complete end-to-end visibility from submission to commercial artwork.
- Global team of regulatory and artwork experts.
- One-stop solution for artwork content to carton operations.
- Accuracy-driven approach to mitigate product recalls.
- Reduced TAT (turnaround time) for faster market entry.
- Consistency in brand and labeling compliance across regions.
- Multi-lingual artwork creation for global markets.