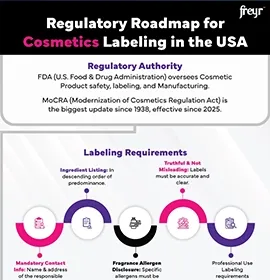
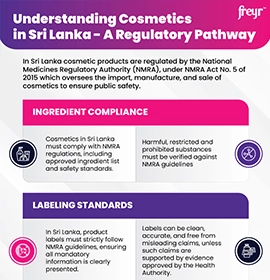
What is Cosmetovigilance?
Cosmetovigilance is defined as the collection, evaluation and monitoring of spontaneous reports of undesirable events including Serious Undesirable Effect (SUE)/ Serious Adverse Effect (SAE) observed during or after normal or reasonably foreseeable use of a cosmetic product.

Why Cosmetovigilance is Important?
- Detecting and addressing adverse reactions, ensuring product safety while reinforcing brand credibility through transparency and a commitment to consumer well-being.
- Reporting Adverse effects of cosmetics and relevant information, documenting, investigating, validating, and assessing these reports to prevent future occurrences. (Learn more about Adverse Event Reporting under MoCRA)
- Conducting safety studies, including the Cosmetic Product Safety Report (CPSR), generating necessary reports, and implementing corrective actions as needed to ensure ongoing product safety. (Learn more about Cosmetic Product Safety Reports)
- Help to meet global regulatory requirements, which prevents costly product recalls and legal consequences.

To view the Market Specific Regulatory Requirements & AE Reporting timelines
Our Cosmetovigilance Services
Our expert services include:

Establish Adverse Events Monitoring Procedure/SOPs
Developing standard operating procedures (SOPs) for effective cosmetovigilance compliance and risk management

Resource Training & Consultation
Providing expert guidance and comprehensive training of resources on regulatory requirements and best practices for cosmetovigilance

Individual Case Safety Report (ICSR) Preparation
End to end case processing (Intake, classification, Cosmetic Safety Data Collection, causality assessment, case narrative preparation, medical review, and serious Cosmetic adverse event reporting to authority).

AE Monitoring & Signal Detection
We track and report any adverse reactions or safety concerns related to your products, ensuring early detection and timely response

Risk Assessment Support
Comprehensive Cosmetic Product Risk Assessment to identify potential safety issues and ensure your products meet all regulatory requirements
Why Choose Freyr?
- Expertise in global regulatory landscapes
- Proactive safety monitoring & compliance strategies
- End-to-end support for regulatory submissions

At Freyr, our cosmetovigilance services are designed to uphold the highest standards of cosmetic product safety and efficacy. Discover how our specialized expertise can help you protect consumer health and ensure continued regulatory compliance.
Frequently Asked Questions (FAQs) on Cosmetovigilance
We are here to provide you with the information you need quickly and efficiently
01. What is cosmetovigilance?
Cosmetovigilance is the ongoing monitoring of the safety of cosmetic products after they are placed on the market, focusing on identifying, assessing, and preventing adverse effects in consumers.
02. Why is cosmetovigilance important?
It helps ensure consumer safety, regulatory compliance, and protects brand credibility by managing risks associated with cosmetic product use.
03. What are the key steps in the cosmetovigilance process to ensure consumer safety?
Cosmetovigilance process is as follows:
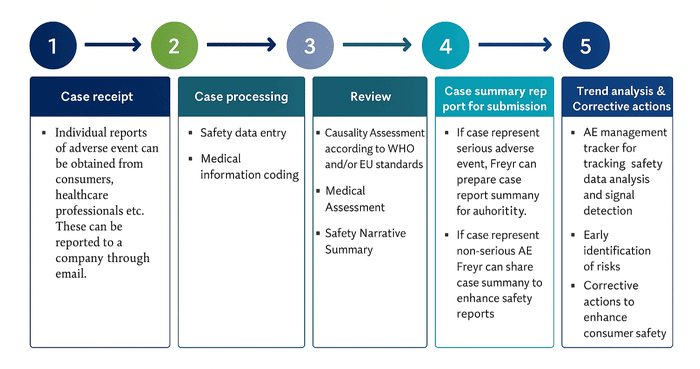
04. Is adverse event reporting mandatory for cosmetics?
Yes, in many regions (e.g., USA under MoCRA, EU under EC No. 1223/2009), reporting adverse events is mandatory. Other countries may have voluntary or conditional requirements.
05. What qualifies as a ‘serious adverse event’?
A serious adverse event includes death, life-threatening experiences, hospitalization, disability, or congenital anomalies due to cosmetic product use.
06. How quickly must adverse events be reported?
Timelines vary by region. Kindly refer to the table provided above.
07. Who is responsible for cosmetovigilance compliance?
The Responsible Person (RP) or manufacturer/importer is accountable for ensuring that adverse events are monitored, recorded, and reported in compliance with local regulations.
08. What is the role of a Cosmetic Product Safety Report (CPSR)?
A CPSR is a mandatory document under EU regulations that assesses the safety of a cosmetic product before it is placed on the market. It includes toxicological profiles, ingredient data, and exposure assessments.
09. How does Freyr support global cosmetovigilance compliance?
Freyr offers end-to-end support including adverse event tracking, regulatory reporting, risk assessment, and preparation of CPSRs tailored to regional requirements.
10. What types of data are collected in cosmetovigilance?
Data includes consumer complaints, clinical trial results, literature reports, and safety concerns raised by health authorities or third-party monitoring systems.
11. Are there penalties for non-compliance in cosmetovigilance?
Yes, failure to comply with cosmetovigilance requirements may lead to product recalls, market withdrawals, regulatory warnings, or financial penalties, depending on the region.
12. How does cosmetovigilance differ from pharmacovigilance?
While both involve safety monitoring, cosmetovigilance is specific to cosmetic products, focusing on non-medical adverse reactions, whereas pharmacovigilance pertains to medicines and therapeutic products.