
According to industry estimates, Regulatory labeling activities account for up to 30% of post-approval Regulatory workloads, while labeling errors also remain among the top contributors to health authority findings, recalls, and delayed approvals. With portfolios expanding global markets, frequent safety updates, and increasing regional divergence, manual and semi-manual labeling processes are no longer sustainable.
Labeling transformation is a Regulatory and business imperative. The key question for life sciences organizations is no longer whether to automate, but where to start.
Why Labeling Automation Is No Longer Optional
Regulatory labeling teams today face mounting pressure from multiple directions:
- Increasing volume of global label variations
- Frequent safety-driven label changes
- Shorter submission timelines
- Heightened inspection and audit scrutiny
- Growing need for CCDS-to-local label alignment
Traditional document-centric workflows rely heavily on spreadsheets, email-based reviews, and manual reconciliation. These methods are prone to version control issues, inconsistent updates, and limited traceability, making audit readiness a constant challenge.
Labeling automation addresses these risks by enabling structured content management, workflow standardization, and real-time visibility across the label lifecycle.
Step 1: Assess Your Current Labeling Landscape
The first step in any labeling transformation roadmap is understanding the current state. Organizations should evaluate:
- A number of products and markets
- Estimate of frequency and complexity of labeling changes
- Degree of manual intervention/efforts across authoring, review, and submission
- Existing integration with Regulatory information management systems
- Audit and inspection pain points
This assessment helps to identify automation-ready areas and prioritize high-impact use cases such as safety updates, variations, and renewals.
Step 2: Standardize Content Before Automating
Automation without standardization only accelerates inefficiencies. Successful labeling transformation starts with content harmonization, particularly at the global level.
Key focus areas include:
- Structuring/Harmonizing Company Core Data Sheets
- Defining reusable content blocks
- Establishing consistent terminology and formatting
- Creating clear governance for content ownership
By standardizing global label content first, organizations can significantly reduce downstream rework across regional and local labels.
Step 3: Introduce Workflow Automation and Governance
Once content is standardized, workflow automation becomes the next critical enabler. Automated workflows replace fragmented email-based processes with role-based, traceable, and compliant review cycles.
Core benefits include:
- Faster review and approval timelines
- Improved version control and change control/tracking
- Built-in audit trails for inspections
- Clear accountability across stakeholders
This step is valuable for organizations to manage high-volume label changes across multiple regions.
Step 4: Enable Regional Labeling at Scale
Regional labeling remains one of the most complex aspects of global Regulatory operations. Each market has unique Regulatory expectations, formats, linguistic requirements, and submission pathways.
Automation supports regional labeling by enabling:
- CCDS-to-local label alignment
- Controlled local adaptations without compromising global consistency
- Parallel processing of regional updates
- Faster response to health authority-driven changes
This approach ensures compliance while maintaining agility in dynamic Regulatory environments.
Step 5: Integrate Labeling with the Regulatory Ecosystem
Labeling does not operate in isolation. For maximum impact, labeling platforms should integrate seamlessly with:
- Regulatory Information Management systems
- Submission and publishing tools
- Safety and pharmacovigilance systems
Integrated ecosystems eliminate data silos, improve consistency across Regulatory activities, and enhance audit readiness.
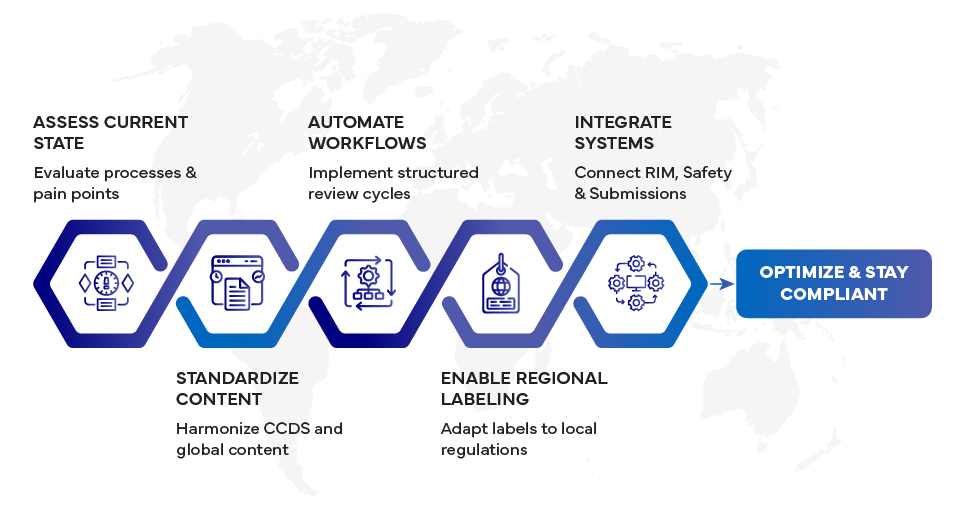
Step 6: Plan for Continuous Optimization
Labeling transformation is not a one-time initiative. Regulatory expectations, technologies, and product portfolios continue to evolve. Mature organizations continuously optimize by:
- Leveraging analytics to identify bottlenecks
- Adopting advanced automation and AI-driven checks
- Refining governance models as portfolios grow
This ensures long-term scalability, compliance, and operational excellence.
Start Smart, Scale with Confidence
Labeling automation is not about replacing Regulatory expertise—it is about empowering teams with smarter tools, standardized processes, and greater control. A well-defined automation roadmap enables organizations to reduce compliance risk, accelerate submissions, and maintain global consistency in an increasingly complex Regulatory landscape.
Freyr supports pharmaceutical and life sciences companies at every stage of their labeling transformation journey. From strategy and content harmonization to advanced labeling automation and regional compliance, Freyr’s Regulatory labeling experts help you build a scalable, inspection-ready labeling ecosystem. Connect with Freyr to accelerate your labeling automation roadmap and achieve global compliance with confidence.









