Introduction: The Shift Toward Patient-First Labeling
As the pharmaceutical landscape evolves, patient-centric labeling is a Regulatory and ethical imperative. Health authorities across regions such as the European Medicines Agency (EMA), the U.S. FDA, and Health Canada are emphasizing the importance of clear, comprehensible, and culturally appropriate product labels. For pharmaceutical companies, this shift demands not just Regulatory compliance but also a deeper understanding of regional nuances, language preferences, and literacy levels to ensure safe and informed use of medicine.
According to industry reports, over 50% of medication errors arise from misinterpreted or unclear labeling information. This statistic underscores why regional labeling must now balance Regulatory precision with patient comprehension, a balance that defines the future of compliant communication in healthcare.
Understanding Regional Labeling Complexities
Every region interprets labeling requirements through its own cultural and Regulatory lens. For example:
- EU ePI initiatives are driving structured digital labeling formats for multilingual access.
- U.S. SPL (Structured Product Labeling) requires standardized XML-based submissions for consistency.
- Asia-Pacific markets often demand translations and adaptations aligned with local dialects and patient literacy levels.
- Latin America imposes evolving artwork, packaging, and serialization mandates that influence regional labeling timelines.
Navigating these dynamic regulations requires integrated regional labeling strategies that align global core data (such as CCDS or CCSI) with local language and content needs, ensuring compliance without compromising clarity.
Patient-Centricity: More Than Just Simplified Language
A truly patient-centric label does more than simplify text; it enhances accessibility, engagement, and trust. This involves:
- Localized language adaptation: Beyond literal translation, labels must reflect local idioms, healthcare norms, and readability standards.
- Cultural sensitivity: Pictograms, color codes, and warnings must be regionally acceptable to prevent misinterpretation.
- Digital enablement: eLabels, QR codes, and mobile-accessible inserts make Regulatory and safety information available in real time.
- Feedback integration: Gathering post-market insights on patient comprehension can guide continuous label optimization.
This holistic approach transforms labeling into an interactive communication tool rather than a static compliance artifact.
Technology as the Enabler of Patient-Centric Labeling
The push toward digital labeling transformation has redefined regional labeling workflows. Advanced tools such as Labeling Management Systems (LMS), Regulatory Information Management (RIM) platforms, and AI-powered translation modules now enable end-to-end visibility, version control, and faster multi-region rollouts.
Automation supports both Regulatory consistency and patient-centric customization by:
- Streamlining CCDS to LPD alignment
- Enforcing labeling traceability across multiple geographies
- Enabling automated quality checks for linguistic accuracy
- Reducing manual intervention and approval bottlenecks
By combining automation with human expertise, pharmaceutical companies can ensure that every label version, global or regional, remains compliant, accurate, and patient-friendly.
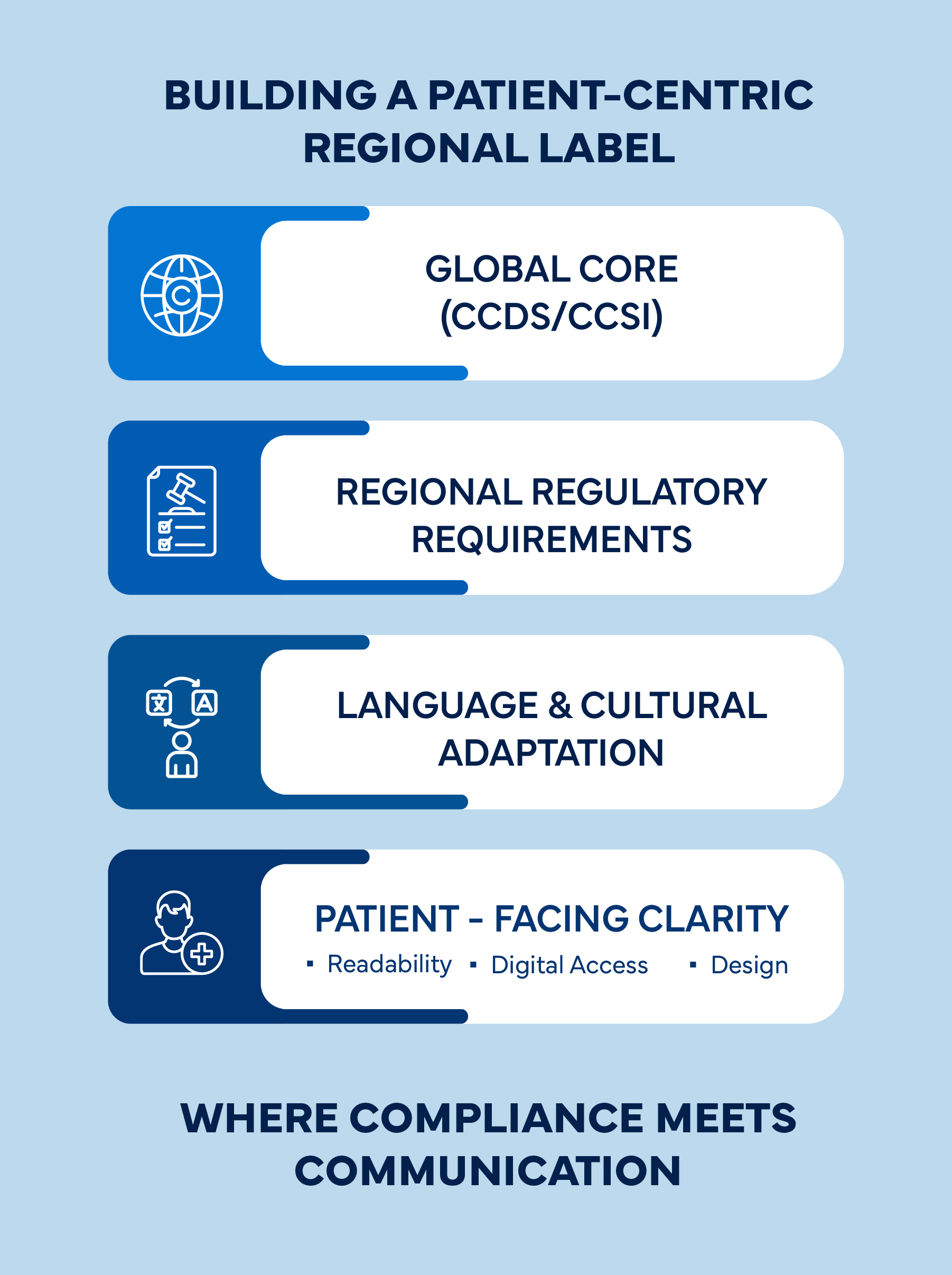
Regulatory Compliance and Clarity: The Delicate Balance
Maintaining compliance while enhancing clarity requires a governance-driven approach. Regulatory teams must establish frameworks that:
- Define a clear CCDS-to-Local Label (LL) process for transparent content flow
- Integrate cross-functional reviews involving Regulatory affairs, medical, quality, and localization experts
- Leverage change control systems to track global and regional label updates
- Adopt standardized templates and style guides to ensure consistency in tone, layout, and readability
Such structures ensure that Regulatory rigor is never compromised, even as labeling becomes more patient-oriented and visually intuitive.
Conclusion
As Regulatory frameworks evolve and patient awareness deepens, the future of pharmaceutical labeling lies in harmonizing compliance with communication clarity. A patient-centric regional labeling approach ensures that every word, symbol, and layout decision contributes to patient safety, adherence, and trust.
Freyr Solutions, with its end-to-end regional labeling services, empowers global pharma companies to navigate complex regional regulations while delivering accurate, compliant, and patient-friendly labels. From CCDS alignment to artwork management and digital labeling transformation — Freyr ensures your labels speak the right language, in every market.
Partner with Freyr Solutions to make your labeling globally compliant and locally understood.









