
Pharmaceutical labeling is one of the most scrutinized aspects of Regulatory compliance. A minor error can have significant consequences for both patient safety and a company's reputation. Consider these findings:
- 20% of pharma and medical device companies report quality control issues with labeling monthly.
- Studies have shown that 40% of medicine labels in certain markets fail to meet Regulatory requirements, often missing key safety information.
- Globally, 35–40% of product recalls stem from packaging and labeling errors, according to US FDA audit summaries.
- In Regulatory audits, over 50% of findings relate to labeling documentation, from prescribing information to artwork and translations.
These statistics underscore one reality: labeling decisions must be precise, evidence-based, and fully justified. The cornerstone of that justification lies in the clinical overview and systematic literature reviews.
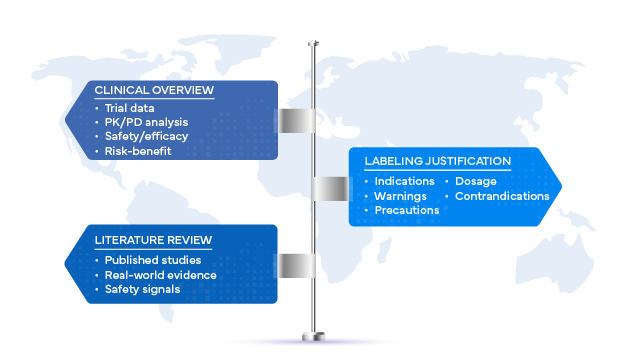
The Role of the Clinical Overview
The clinical overview, as defined by ICH M4E(R2), is more than a Regulatory formality. It is a structured, critical analysis of all available clinical data supporting the development of a product. When tied to labeling, it plays a decisive role in ensuring that the safety, efficacy, and risk–benefit profile are clearly represented in prescribing information.
Key contributions of the clinical overview to labeling include:
- Mapping clinical trial outcomes directly to product claims
- Justifying the inclusion of warnings, precautions, and contraindications
- Ensuring consistency between safety findings and labeling decisions
- Presenting regulators with a transparent, evidence-driven benefit–risk analysis
For example, if trial data reveal a statistically significant adverse event in a subgroup, the clinical overview supports the rationale for including subgroup-specific warnings in the label.
Why Literature Reviews Are Essential
While clinical trial data form the foundation of labeling justification, systematic literature reviews expand the evidence base by incorporating findings beyond sponsor-run studies. These reviews synthesize peer-reviewed publications, real-world evidence, and post-marketing safety data to provide regulators with a broader, unbiased perspective.
A strong literature review helps companies to:
- Validate safety claims through independent sources
- Detect early warning signals or adverse reactions not evident in clinical trials
- Benchmark findings against competitor products or class-wide effects
- Harmonize Core Data Sheets (CCDS) with region-specific Local Product Documents (LPD)
For example, published studies or meta-analyses may reveal rare but serious adverse events, strengthening the justification for including or updating a precaution in the label.
Regulatory Expectations for Labeling Justification
Global health authorities, including the US FDA, EMA, MHRA, and PMDA, expect labeling justifications to reflect scientific rigor, transparency, and consistency. Regulatory reviewers often evaluate:
- Evidence hierarchy: Prioritizing randomized controlled trial data but acknowledging relevant observational or real-world studies
- Cross-document consistency: Ensuring that the clinical overview, Summary of Clinical Safety (SCS), and proposed label align
- Clear rationale: Showing why each indication, dosage recommendation, or warning is included (or excluded)
Failure to provide a clear evidence base for labeling can result in Regulatory queries, extended review timelines, or even rejection.
Best Practices for Justifying Labeling Decisions
Pharmaceutical companies can strengthen their labeling submissions by adopting these best practices:
- Cross-functional collaboration: Engage clinical, safety, and Regulatory teams for a holistic interpretation
- Leverage technology: Use AI-enabled tools for literature reviews to accelerate screening and extraction
- Maintain documentation trails: Record inclusion/exclusion rationales for studies to satisfy Regulatory audits
- Benchmark globally: Align with competitor products and evolving guidelines to close compliance gaps
- Update continuously: Incorporate emerging literature and pharmacovigilance data into ongoing labeling strategies
Conclusion
With Regulatory scrutiny at an all-time high and the cost of non-compliance increasing, companies cannot afford to rely on assumptions when drafting product labels. A robust clinical overview combined with comprehensive literature reviews ensures that every claim is backed by transparent, scientific evidence.
At Freyr Solutions, we help global pharma organizations navigate this complexity. Our end-to-end expertise in Regulatory labeling, clinical documentation, and compliance strategies ensures your labeling decisions are scientifically justified, regulator-ready, and globally aligned.
Partner with Freyr today to strengthen your labeling strategy with evidence-based justifications that regulators trust and patients depend on.









