
In today’s fast-paced pharmaceutical landscape, automation is a Regulatory necessity. According to recent industry reports, over 60% of pharma labeling errors stem from manual processes, and nearly 70% of companies cite labeling delays as a major cause of postponed product launches. Moreover, the global pharmaceutical labeling automation market is projected to grow at a CAGR of over 10% through 2030, driven by increasing Regulatory complexity and the shift toward digital labeling ecosystems.
These figures underscore a crucial truth — as labeling requirements evolve, partnering for labeling automation isn’t just about keeping up; it’s about staying ahead.
The Complexity Challenge: Why Automation Matters
Regulatory labeling today involves more than text and templates. It’s about managing dynamic, multi-market content across dozens of global health authorities, each with unique rules and formats. From CCDS-to-LPD alignment and multilingual translations to safety updates and digital eLabeling, the scope is vast and constantly evolving.
Manual or siloed approaches struggle to handle such complexity. Inconsistent updates, version control errors, and communication gaps can lead to compliance breaches, delayed approvals, and costly recalls. This is where labeling automation, supported by an experienced Regulatory partner, delivers both strategic and operational value.
Partnering for Labeling Automation: A Strategic Move
Implementing labeling automation isn’t a plug-and-play exercise. It demands a deep understanding of Regulatory frameworks, data governance, content workflows, and system integration — all of which benefit from expert collaboration. Partnering with a specialized Regulatory technology provider like Freyr Solutions offers multiple strategic advantages:
Regulatory Expertise Meets Automation Intelligence
Freyr combines domain expertise with technology fluency. This ensures that automation tools are configured in line with global and regional Regulatory requirements, not just IT standards. From FDA’s SPL to EMA’s ePI and Health Canada’s XML standards, Freyr ensures every automation layer is compliant by design.
End-to-End Labeling Ecosystem Integration
A robust partner doesn’t just automate workflows — they connect the dots between Regulatory Information Management (RIM), Artwork Management Systems, and Enterprise Labeling Solutions. This enables a single source of truth, improving visibility, accuracy, and collaboration across functions like Regulatory Affairs, QA, and Manufacturing.
Scalable, Future-Ready Solutions
As pharma labeling moves toward digital-first formats, an automation partner helps organizations scale seamlessly — whether adding new markets, adapting to health authority updates, or integrating new technologies like AI for content validation and comparison.
Reduced Time-to-Market and Error Rates
With automated data flows and version-controlled content libraries, companies experience up to 50% reduction in labeling cycle times and drastically fewer manual errors. This translates directly into faster product launches and stronger compliance performance.
Strategic Resource Optimization
By outsourcing automation strategy and operations, Regulatory teams can focus on high-value decision-making instead of repetitive administrative tasks. The right partner ensures smooth change management, system training, and process governance.
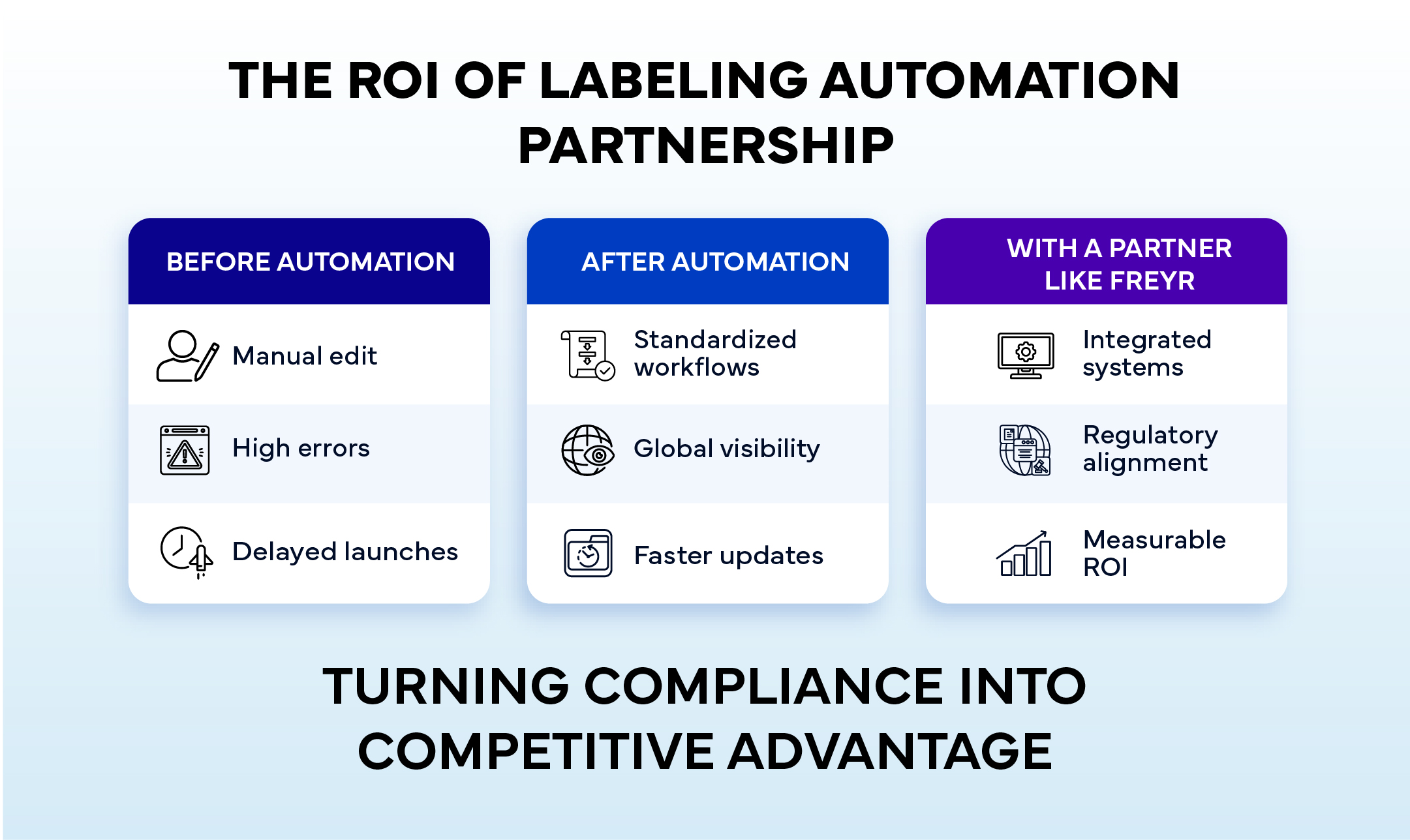
Technology-Driven Compliance: The Freyr Approach
Freyr’s Labeling Automation services are built on a science-driven framework that blends Regulatory intelligence with digital innovation. The approach includes:
- Gap assessment and roadmap design to identify automation opportunities.
- Template standardization for consistent CCDS, SmPC, and PIL content.
- Automated change management workflows ensuring timely updates across markets.
- AI-powered comparison tools to ensure alignment across CCDS, local labels, and artwork.
- Seamless integration with existing RIM, PLM, and document management systems.
By deploying automation strategically, Freyr helps clients achieve compliance continuity, faster turnaround times, and error-proof label governance.
Transforming Labeling from Compliance to Competitive Edge
In an era where speed, accuracy, and compliance define market success, labeling automation is no longer optional — it’s strategic. Partnering with a trusted provider ensures that automation delivers both technological efficiency and Regulatory confidence. Freyr Solutions stands at the intersection of technology, Regulatory expertise, and innovation, empowering pharmaceutical companies to future-proof their labeling operations.
Accelerate your compliance journey. Partner with Freyr Solutions to automate, optimize, and transform your global labeling ecosystem.









