
Effective communication has always ensured the safety and quality of care for patients. Over the years, there remains an unmet requirement of providing label translations to individuals who are not fluent in English. The demand for product labels in multiple regional languages has grown exponentially as pharmaceutical manufacturers intend to drive the change towards localization and better communication.
Fulfilling these demands will enhance patient safety while enabling compliance with country-specific Regulatory requirements. The absence of important medical information in regional scripts puts an additional burden on pharmacists and healthcare professionals to address the language barrier.
The Health Products Regulatory Authority (HPRA) overlooks Regulatory compliance in Ireland. The HPRA facilitates and jointly works with the stakeholders by adhering to the best practice guidance on multilingual labeling released by the Coordination Group for Mutual Recognition and Decentralised Procedures – Human (CMDh). In addition, the HPRA also relaxed certain labeling conditions, which include:
- Use of mL in Ireland (IE) versus ml in other EU states.
- Use of decimal points in the product volume or strength and a dot in IE versus comma in the EU. Drug manufacturers can overcome this discrepancy by grouping the invented name, strength, and drug form as one unit in each language.
- Use of separator in the product strength or volume - A dot in EU versus a comma in IE. The use of commas as a separator is allowed on the immediate outer package to avoid any risk of confusion in the Irish patient population.
- Small immediate packaging units, i.e., containers with size lesser or equal to 50ml, have a constraint of space and can accommodate minimum required information on them, wherever justified, for more than two (02) languages.
- Third country information – wherever the conditions of product information are similar in the UK and IE, multi-country packs are acceptable. Additional country-specific requirements are placed in a ‘blue box.’
- Coordinating assessment with the other Member States - Mock-up assessments carried out by the Member States ensure labeling compliance. Applicants can coordinate with the Member States to get clarity on the changes required before the submissions to the HPRA.
- Joint names - Highlighting of the invented name prop to the Member States must be informed to the HPRA.
HPRA Puts Forth Additional Suggestions for Multilingual Labeling as Follows:
- Information mentioned in English must be grouped in a block.
- Mention ‘blue-box’ requirements for all the countries on the same panel wherever several countries share one pack.
- Information directed towards the Irish patients must be clearly stated in an oval code.
- Applicants can include a perforated section on the pack, provided that the removable data is not in English. This must be mentioned in the submission.
- Applicants must submit a PDF of the package leaflet to the HPRA at the end of the process for updating it on the website.
Criteria for a Multilingual Package:
- The product name and its strength must be same in all the languages.
- The labeling and package leaflet information must be in accordance with the Summaries of Product Characteristics (SmPC).
- Information printed on the labeling and package leaflet must be accurate and legible.
- The legal status of the drug must be similar in both the countries.
An example of multilingual packaging would be:
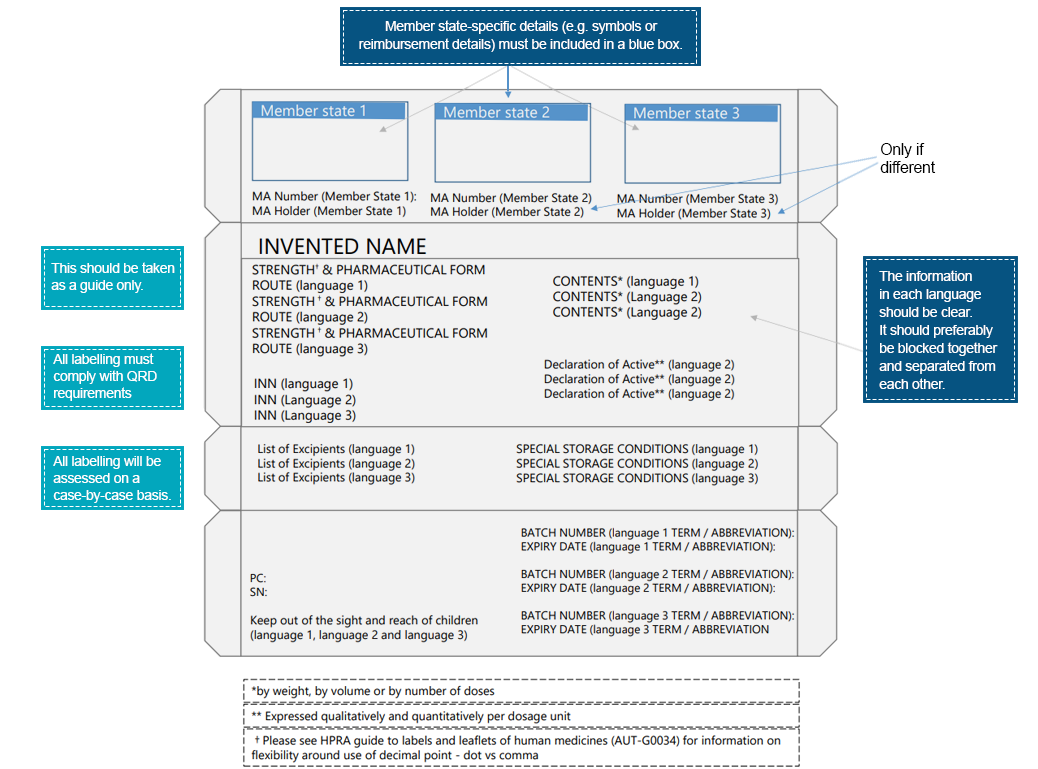
Patients interact with the drug labels way before they interact with the drug product. Such interactions further highlight the significance of conveying essential information across various elements in the drug label. To ensure better readability and improvisation of label elements, pharmaceutical companies must undertake necessary measures. Regional languages provide a medium to convey medical instruction relevant to the drug product that promises the safe and effective use of the prescribed product. Country-specific Regulatory experts can assist with translating labeling information in compliance with local/regional regulations. Freyr experts are well-equipped to provide assistance with bridging gaps between medical information and language. Reach out to us for a compliant labeling pathway.









